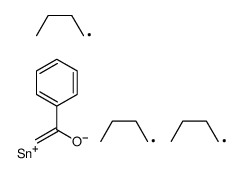
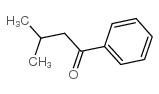
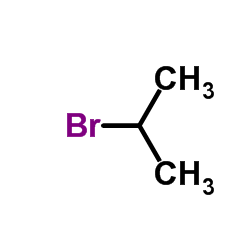
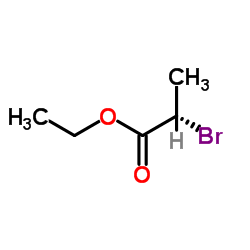
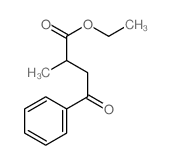
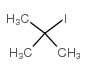
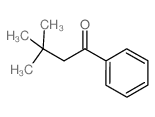
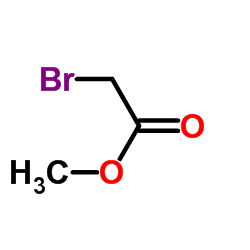
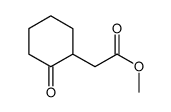
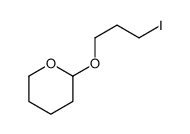
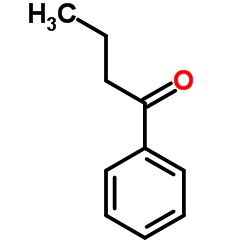
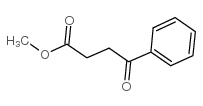
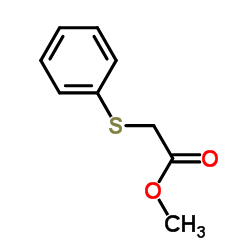
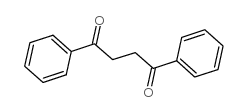
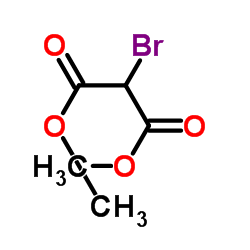
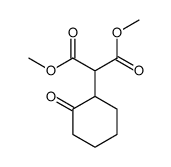
|
~99% |
|
~89% |
|
~63% |
|
~92% |
|
~99% |
|
~90% |
|
~% |
|
~79% |
|
~93% |
|
~43% |
|
~94% |
|
~84% |
|
~94% |
|
~94% |