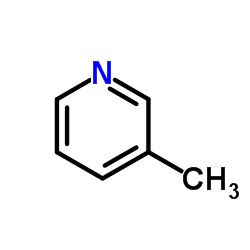
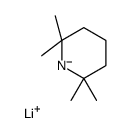
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
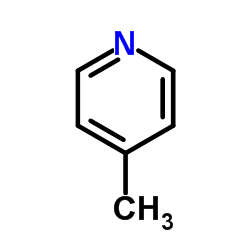
![[tert-butyl(trimethylsilyl)amino]lithium结构式](https://image.chemsrc.com/caspic/077/18270-42-3.png)
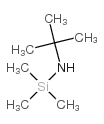
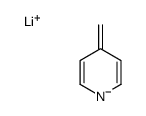
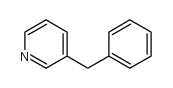
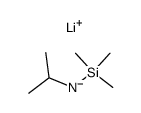



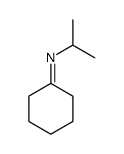

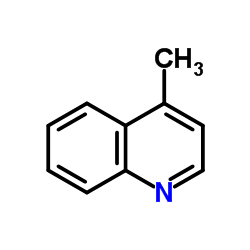

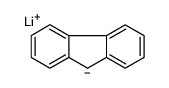
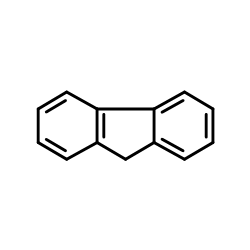
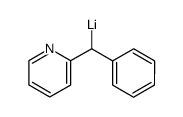
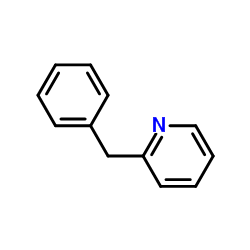
![[(pyridin-4-yl)phenylmethyl]lithium结构式](https://image.chemsrc.com/caspic/048/81771-00-8.png)
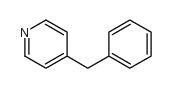
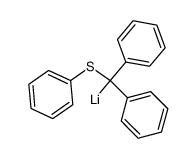
![Benzene,1,1'-[(phenylthio)methylene]bis结构式](https://image.chemsrc.com/caspic/279/21122-20-3.png)