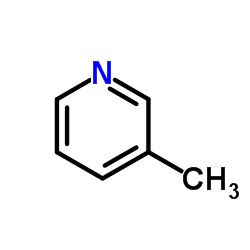
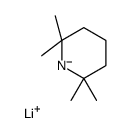
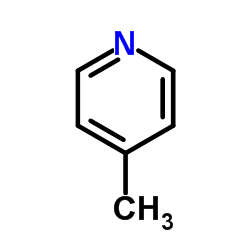
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
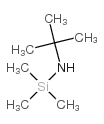
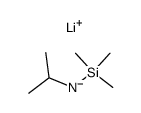
![[tert-butyl(trimethylsilyl)amino]lithium Structure](https://image.chemsrc.com/caspic/077/18270-42-3.png)
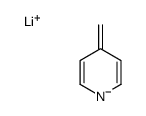
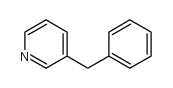



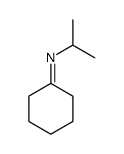

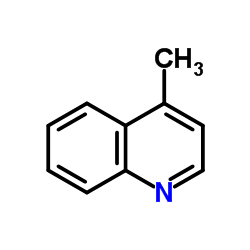

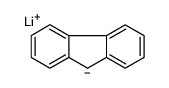
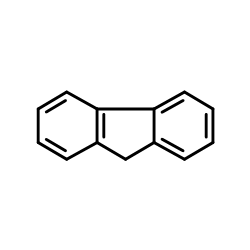
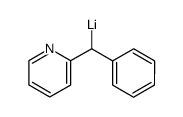
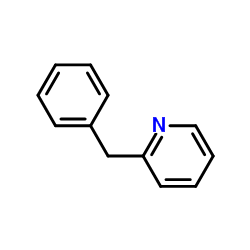
![[(pyridin-4-yl)phenylmethyl]lithium Structure](https://image.chemsrc.com/caspic/048/81771-00-8.png)
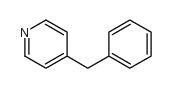
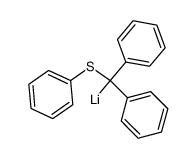
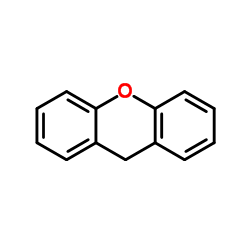
![Benzene,1,1'-[(phenylthio)methylene]bis Structure](https://image.chemsrc.com/caspic/279/21122-20-3.png)