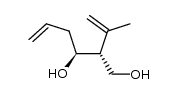
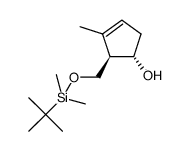
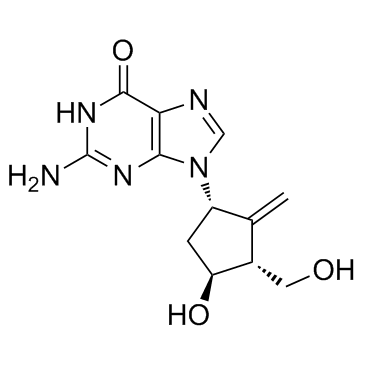
|
~93% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~87% |

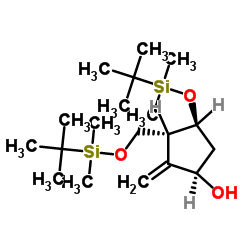
![(1S,2R,3S,5R)-2-(((tert-butyldimethylsilyl)oxy)methyl)-1-methyl-6-oxabicyclo[3.1.0]hexan-3-ol结构式](https://image.chemsrc.com/caspic/086/1384268-93-2.png)