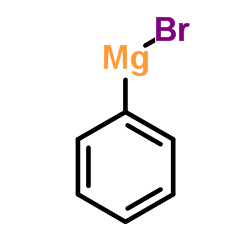
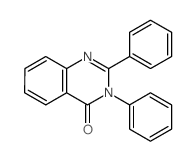
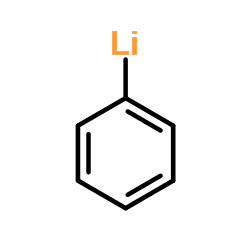
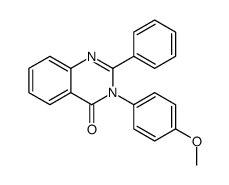
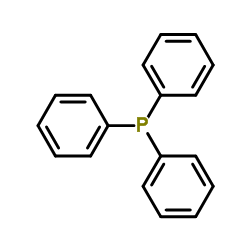
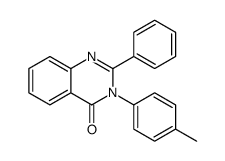
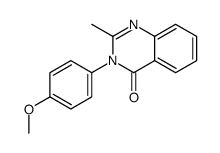
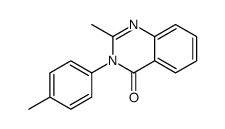
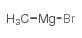|
~68% |
|
~% |
|
~% |
|
~96% |
|
~33% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~89% |
|
~% |
|
~% |
|
~92% |
|
~81% |
|
~49% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |


![ethyl 2-[(triphenyl-λ5-phosphanylidene)amino]benzoate结构式](https://image.chemsrc.com/caspic/218/179039-70-4.png)