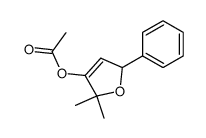
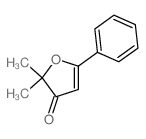
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~14% |
|
~% |
|
~% |
|
~% |
|
~10% |
|
~% |
|
~96% |
|
~% |
|
~% |
|
~% |
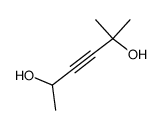

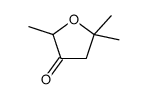
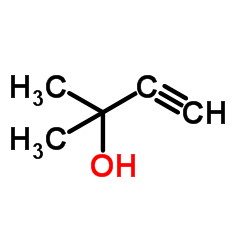

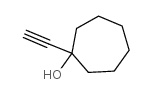
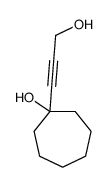
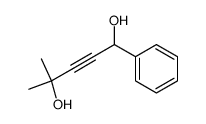


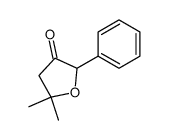



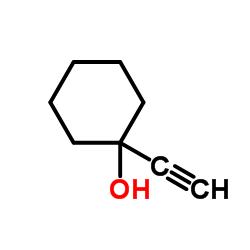

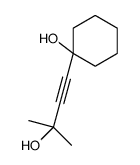
![3-acetoxy-5-[(E)-3-(2-oxo-2H-chromen-7-yloxy)-1-methyl-1-propenyl]-2,2-dimethyl-2,5-dihydrofuran结构式](https://image.chemsrc.com/caspic/033/78957-41-2.png)
![2H-1-Benzopyran-2-one,7-[[(2E)-3-(4,5-dihydro-5,5-dimethyl-4-oxo-2-furanyl)-2-buten-1-yl]oxy]结构式](https://image.chemsrc.com/caspic/234/36413-91-9.png)