|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![(1S,2R,4R)-1-((N,N-dicyclohexylsulfamoyl)methyl)-7,7-dimethylbicyclo[2.2.1]heptan-2-yl (S)-3-methylheptanoate结构式](https://image.chemsrc.com/caspic/424/96864-22-1.png)


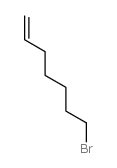
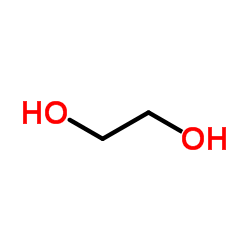
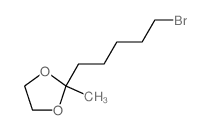
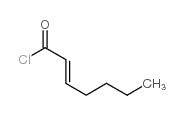

![(1S,2R,4R)-1-((N,N-dicyclohexylsulfamoyl)methyl)-7,7-dimethylbicyclo[2.2.1]heptan-2-yl (R)-3-methylheptanoate结构式](https://image.chemsrc.com/caspic/272/96864-26-5.png)