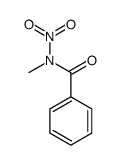
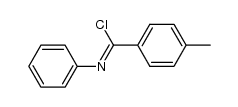
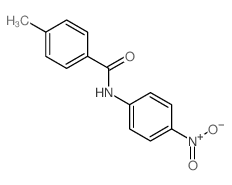
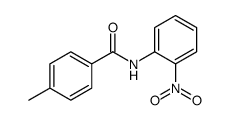
|
~% |
|
~42% |
|
~% |
|
~% |