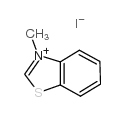
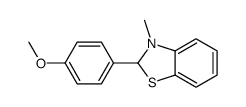
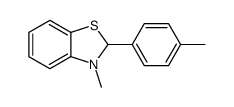
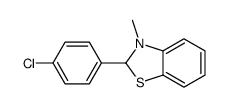
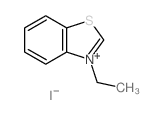
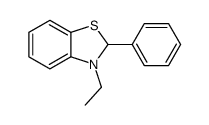
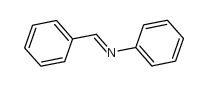
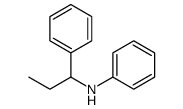
|
~34% |
|
~68% |
|
~56% |
|
~33% |
|
~% |