|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
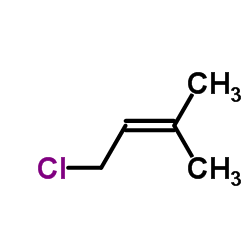

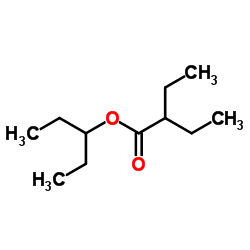
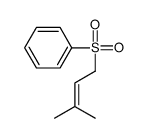
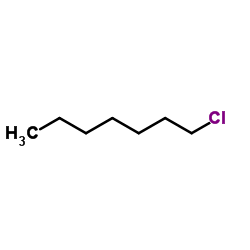


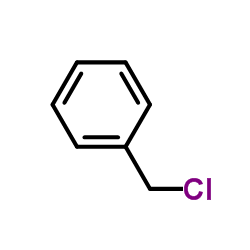
![Benzene, [(1Z)-2-bromoethenyl]结构式](https://image.chemsrc.com/caspic/491/588-73-8.png)
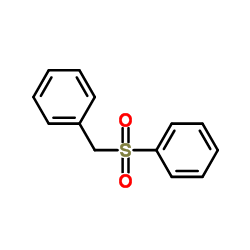
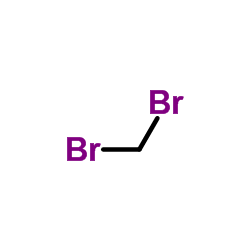
![Benzene, [(1E)-2-bromoethenyl]结构式](https://image.chemsrc.com/caspic/029/588-72-7.png)
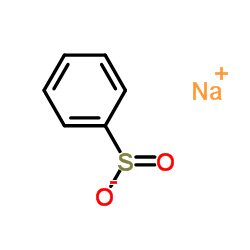
![Benzene,[(1-phenylethyl)sulfonyl]结构式](https://image.chemsrc.com/caspic/039/24422-78-4.png)
