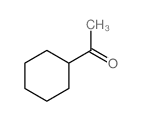
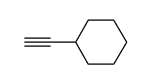
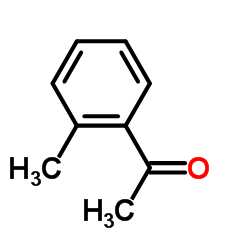
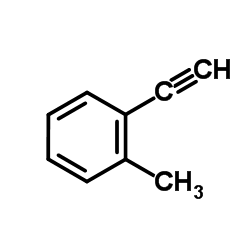
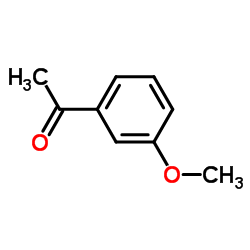
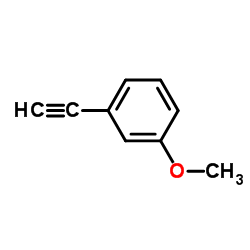
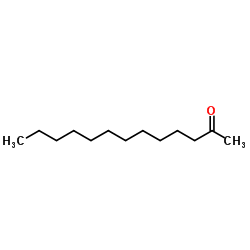
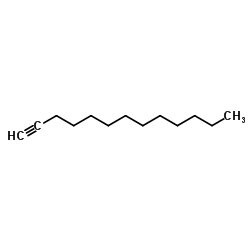
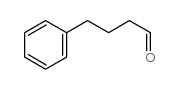
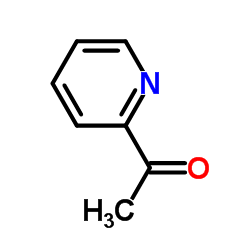
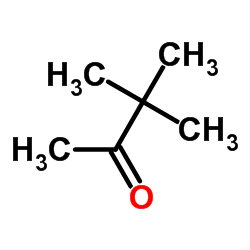
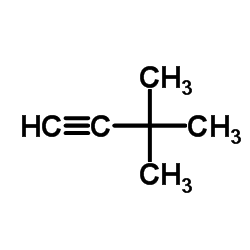
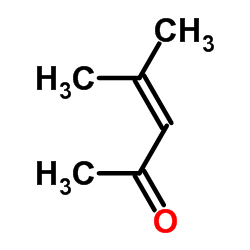
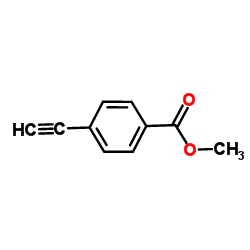
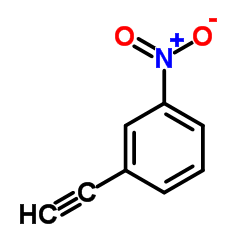
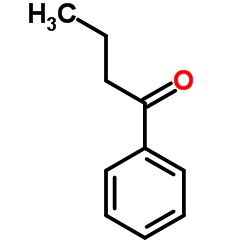
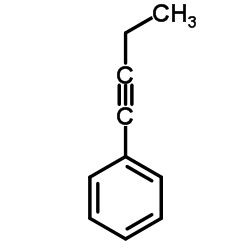
|
~54% |
|
~64% |
|
~78% |
|
~78% |
|
~77% |
|
~% |
|
~% |
|
~88% |
|
~64% |
|
~59% |
|
~53% |
|
~97% |
|
~78% |
|
~96% |
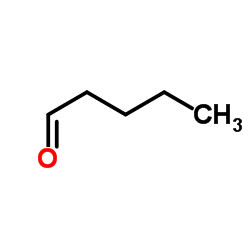
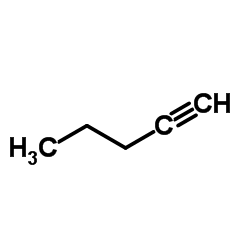
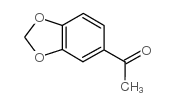
![5-炔基苯并[d][1,3]二氧杂环戊烯结构式](https://image.chemsrc.com/caspic/119/57134-53-9.png)