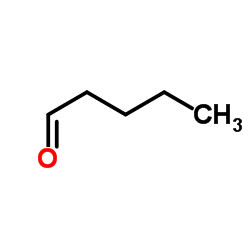
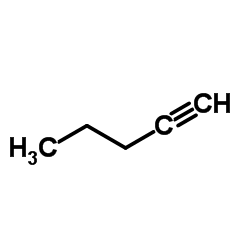
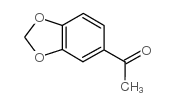
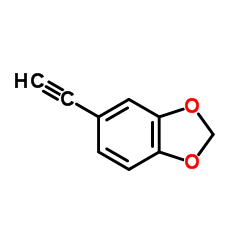
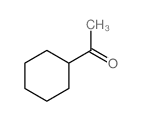
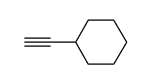
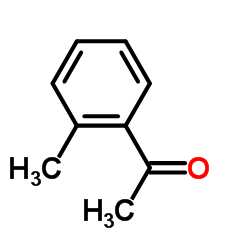
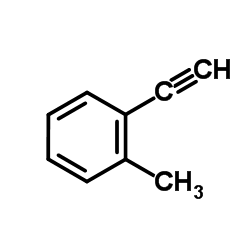
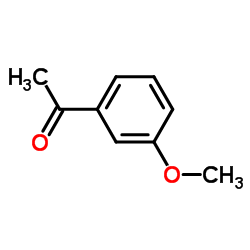
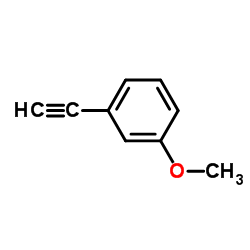
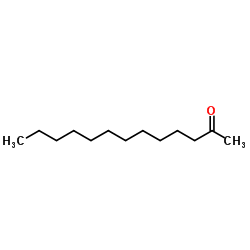
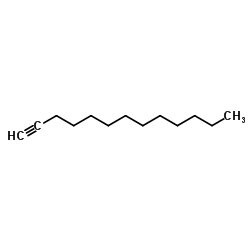
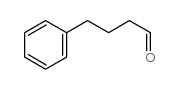
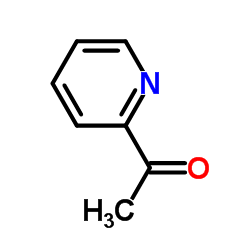
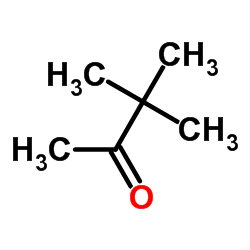
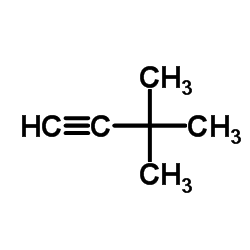
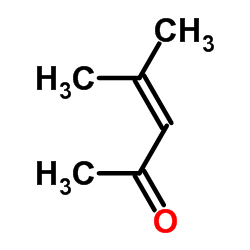
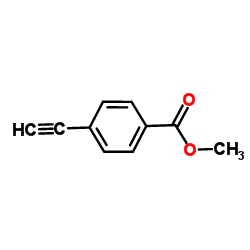
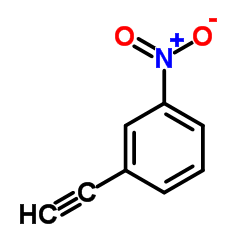
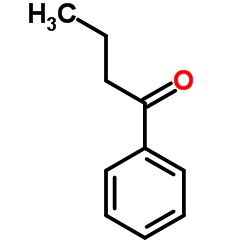
|
~54% |
|
~64% |
|
~78% |
|
~78% |
|
~77% |
|
~% |
|
~% |
|
~88% |
|
~64% |
|
~59% |
|
~53% |
|
~97% |
|
~78% |
|
~96% |