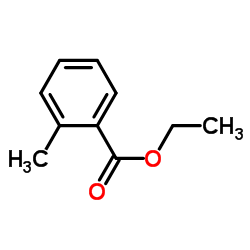
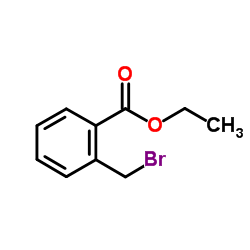
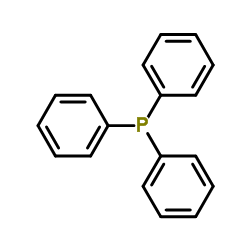
|
~85% |
|
~% |
|
~% |
|
~93% |
|
~% |

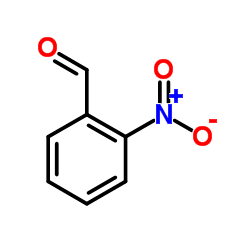
![ethyl 2-[2-(2-nitrophenyl)ethenyl]benzoate结构式](https://image.chemsrc.com/caspic/130/90011-52-2.png)