|
~84% |
|
~92% |
|
~90% |
|
~83% |
|
~57% |
|
~84% |
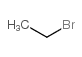
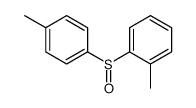
![1-[(S)-(4-methylphenyl)sulfinyl]-2-propylbenzene结构式](https://image.chemsrc.com/caspic/455/835626-63-6.png)


![1-[(S)-(4-methylphenyl)sulfinyl]-2-(2-phenylethyl)benzene结构式](https://image.chemsrc.com/caspic/417/835626-64-7.png)
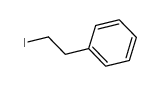
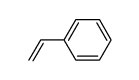
![1-[(S)-(4-methylphenyl)sulfinyl]-2-(3-phenylpropyl)benzene结构式](https://image.chemsrc.com/caspic/379/835626-65-8.png)

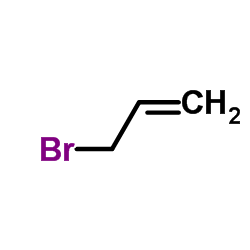
![1-but-3-enyl-2-[(S)-(4-methylphenyl)sulfinyl]benzene结构式](https://image.chemsrc.com/caspic/293/835626-67-0.png)