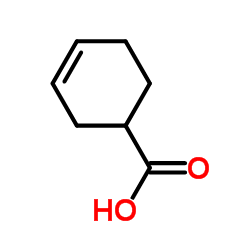
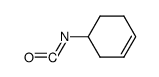
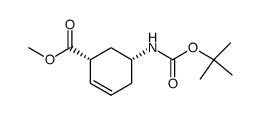
|
~87% |
|
~% |
|
~69% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~51% |
|
~% |

![6-Azabicyclo[3.2.1]oct-2-en-7-one(9CI)结构式](https://image.chemsrc.com/caspic/455/89622-07-1.png)