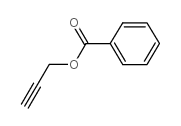
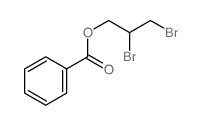
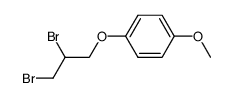
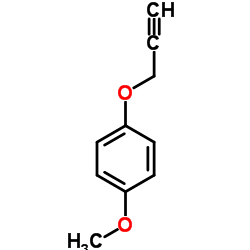
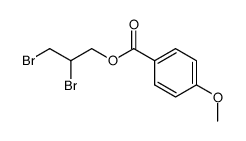
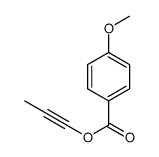
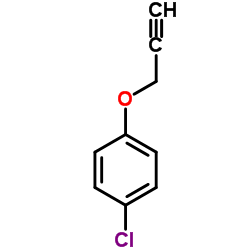
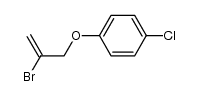
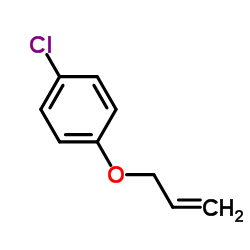
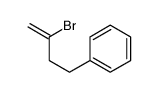
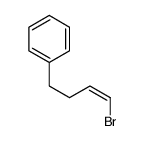
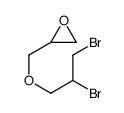
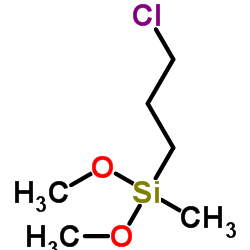
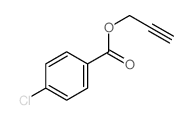
|
~56% |
|
~40% |
|
~86% |
|
~93% |
|
~78% |
|
~83% |
|
~84% |
|
~87% |
|
~% |
|
~45% |
|
~70% |
|
~43% |
|
~9% |