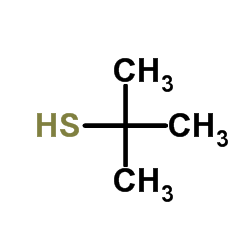
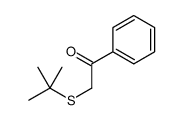
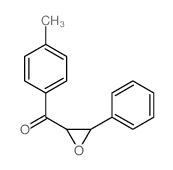
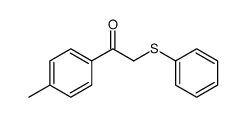
|
~90% |
|
~79% |
|
~89% |
|
~88% |
|
~78% |
|
~85% |
|
~90% |
|
~88% |
|
~82% |
|
~77% |
|
~90% |
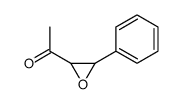

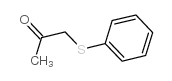
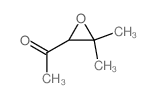
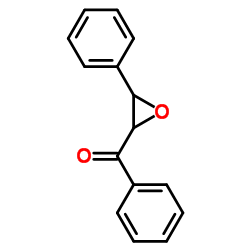
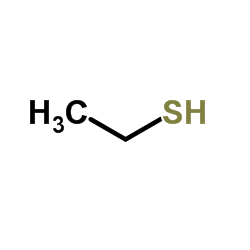
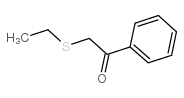
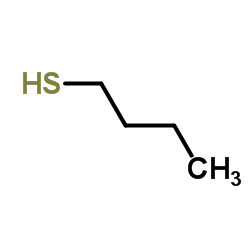
![2-(4-bromophenyl)-N-[1-[(4-methylphenyl)methyl]pyrazol-3-yl]acetamide结构式](https://image.chemsrc.com/caspic/067/6306-17-8.png)
![Methanone,[3-(4-chlorophenyl)-2-oxiranyl]phenyl结构式](https://image.chemsrc.com/caspic/370/6969-01-3.png)
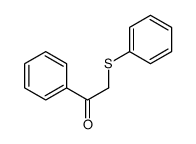
![[3-(4-methoxyphenyl)oxiran-2-yl]-phenyl-methanone结构式](https://image.chemsrc.com/caspic/332/6969-02-4.png)
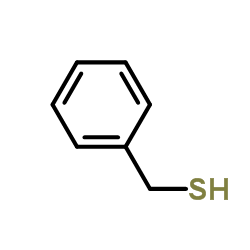
![Ethanone,1-phenyl-2-[(phenylmethyl)thio]结构式](https://image.chemsrc.com/caspic/454/2408-88-0.png)