|
~% |
|
~% |
|
~% |
|
~88% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![(2'R,3R,4S)-5-[2'-methoxy-2'-(trifluoromethyl)phenylacetoxy]-2,3,4-trimethyl-3,4-methylenedioxy-2-pentene结构式](https://image.chemsrc.com/caspic/082/121719-94-6.png)
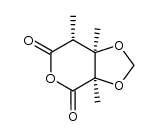
![(2'R,2S,3R,4S)-5-[2'-methoxy-2'-(trifluoromethyl)phenylacetoxy]-2,3,4-trimethyl-3,4-methylenedioxypentanoic acid结构式](https://image.chemsrc.com/caspic/006/121719-96-8.png)
![(2'R,3R,4S)-5-[2'-methoxy-2'-(trifluoromethyl)phenylacetoxy]-2,3,4-trimethyl-3,4-methylenedioxy-2-pentanol结构式](https://image.chemsrc.com/caspic/120/121719-93-5.png)
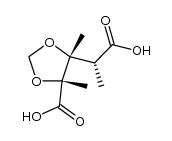


![(2'R,2R,3R,4S)-5-[2'-methoxy-2'-(trifluoromethyl)phenylacetoxy]-2,3,4-trimethyl-3,4-methylenedioxy-1-pentanol结构式](https://image.chemsrc.com/caspic/044/121719-95-7.png)