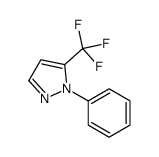
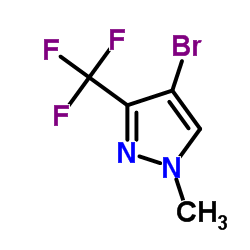
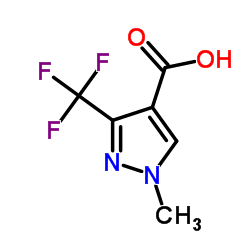
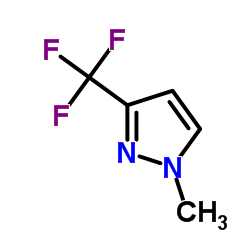
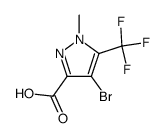
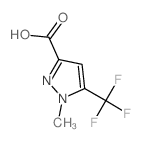
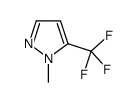
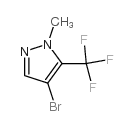
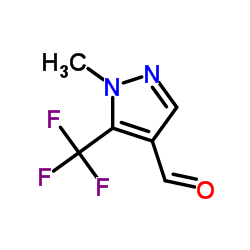
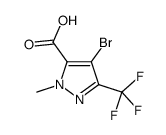
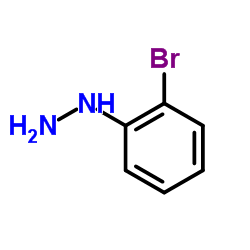
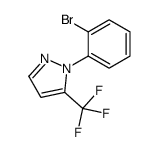
|
~60% |
|
~85% |
|
~71% |
|
~93% |
|
~67% |
|
~5% |
|
~% |
|
~% |
|
~64% |
|
~66% |
|
~% |
|
~88% |
|
~% |
|
~80% |
|
~13% |
|
~% |
|
~% |
|
~48% |
|
~78% |
|
~25% |