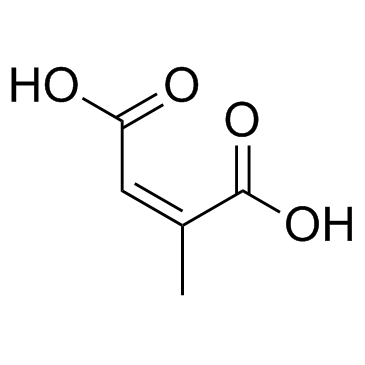
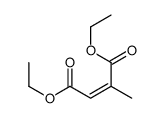
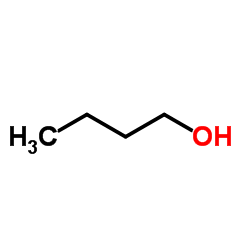
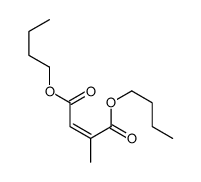
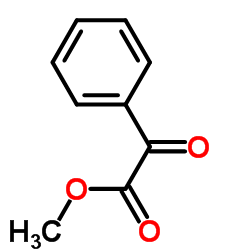
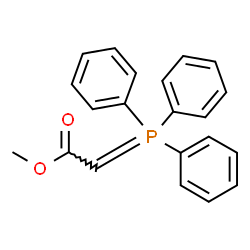
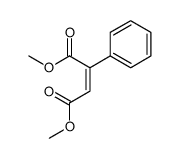
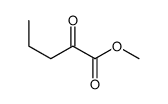
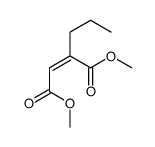
|
~88% |
|
~80% |
|
~33% |
|
~23% |