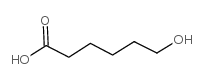
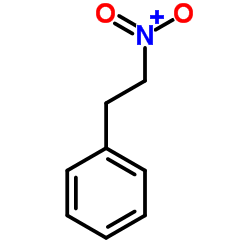
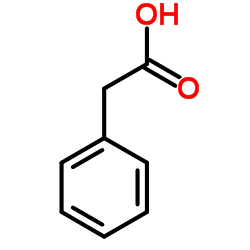
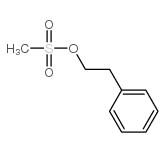
|
~72% |
|
~95% |
|
~87% |
|
~44% |
|
~88% |
|
~87% |