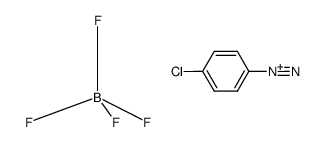
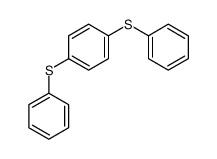
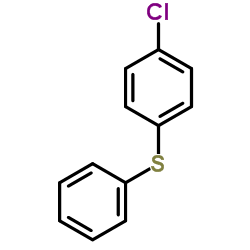
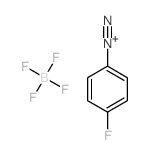
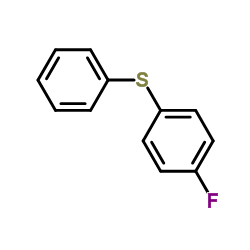
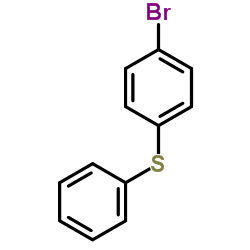
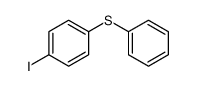
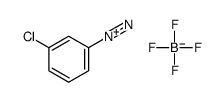
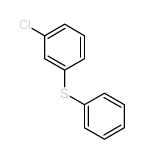
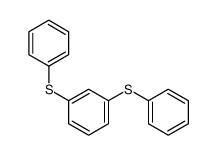
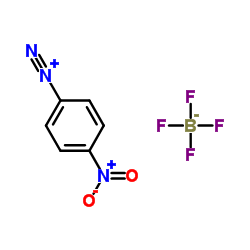
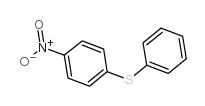
|
~65% |
|
~0% |
|
~68% |
|
~64% |
|
~61% |
|
~13% |
|
~91% |
|
~80% |