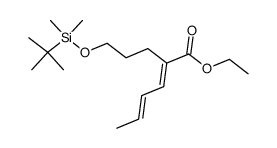
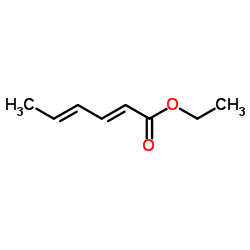
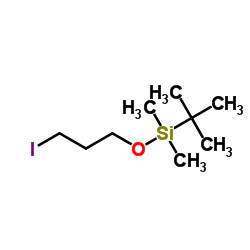
|
~69% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

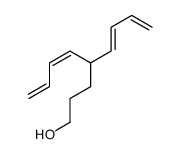
![2-[3-[tert-butyl(dimethyl)silyl]oxypropyl]hexa-3,5-dienal结构式](https://image.chemsrc.com/caspic/417/922515-78-4.png)
![(E)-2-[3-(tert-Butyl-dimethyl-silanyloxy)-propyl]-hexa-3,5-dienoic acid ethyl ester结构式](https://image.chemsrc.com/caspic/069/922515-74-0.png)