|
~69% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

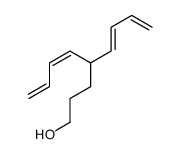
![2-[3-[tert-butyl(dimethyl)silyl]oxypropyl]hexa-3,5-dienal Structure](https://image.chemsrc.com/caspic/417/922515-78-4.png)
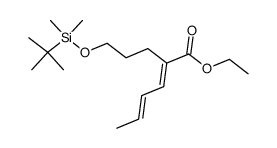
![(E)-2-[3-(tert-Butyl-dimethyl-silanyloxy)-propyl]-hexa-3,5-dienoic acid ethyl ester Structure](https://image.chemsrc.com/caspic/069/922515-74-0.png)

![1-Iodo-3-[(tert-butyldimethylsilyl)oxy]propane Structure](https://image.chemsrc.com/caspic/393/78878-05-4.png)