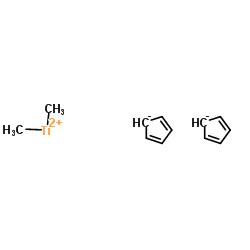
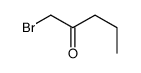
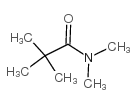
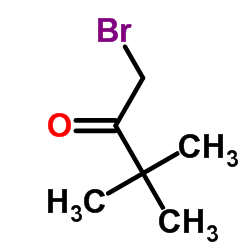
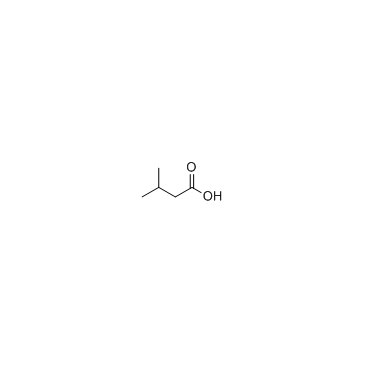
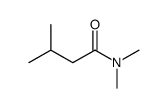
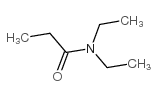
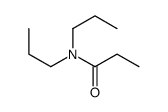
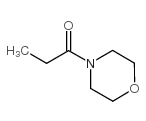
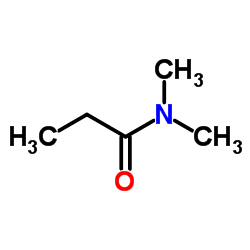
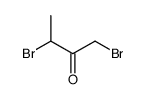
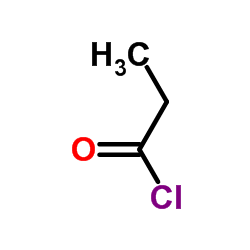
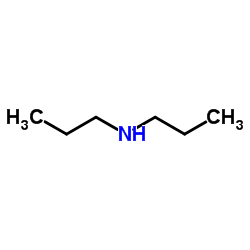
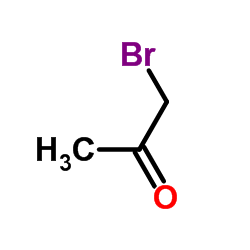
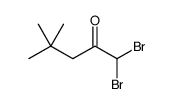
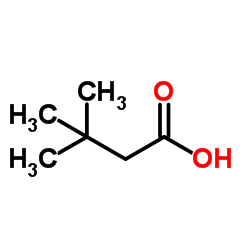
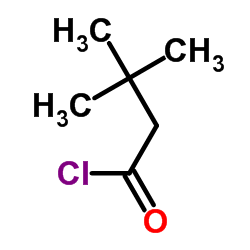
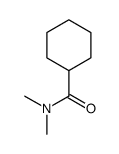
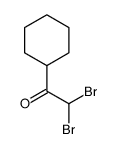
|
~90% |
|
~97% |
|
~% |
|
~% |
|
~88% |
|
~93% |
|
~97% |
|
~89% |
|
~93% |
|
~94% |
|
~35% |
|
~0% |
|
~% |
|
~87% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |