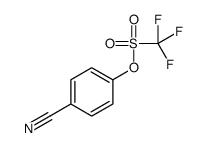
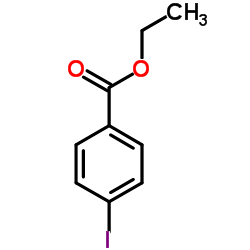
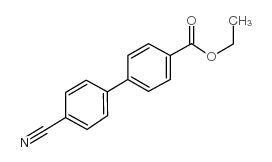
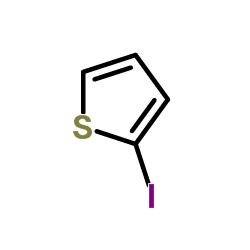
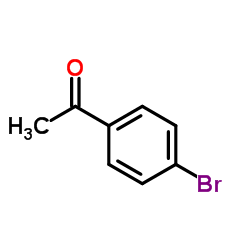
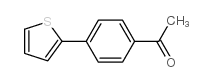
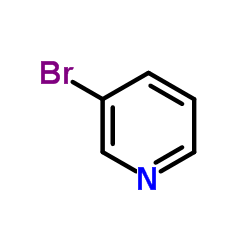
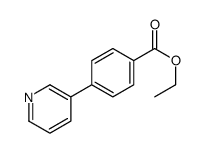
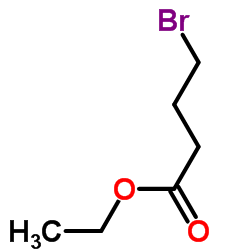
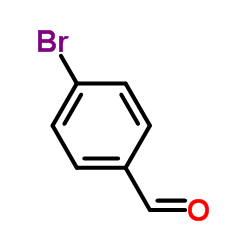
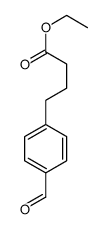
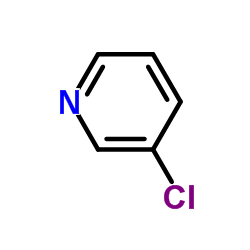
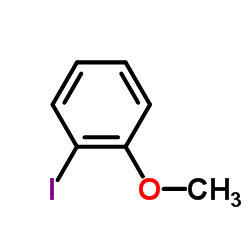
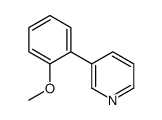
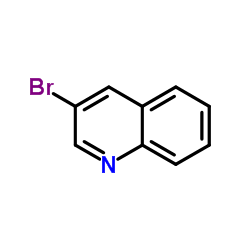
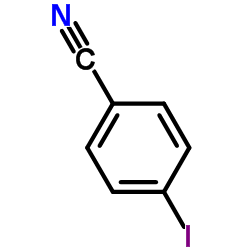
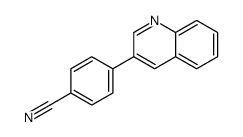
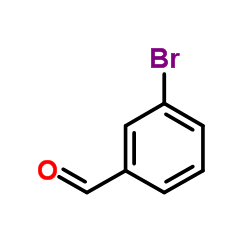
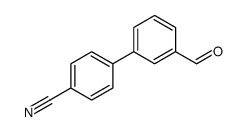
|
~77% |
|
~82% |
|
~83% |
|
~87% |
|
~94% |
|
~91% |
|
~79% |