|
~96% |
|
~% |
|
~71% |
|
~% |
|
~10% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~96% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~87% |
|
~88% |
|
~73% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~77% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~87% |
|
~% |
|
~% |
|
~% |

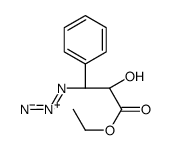
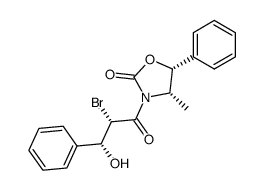
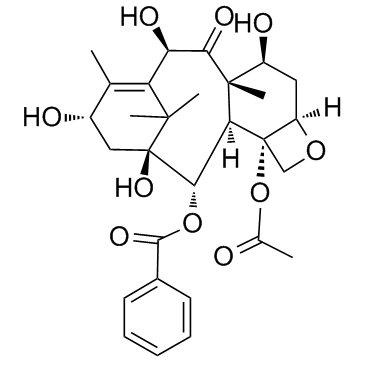

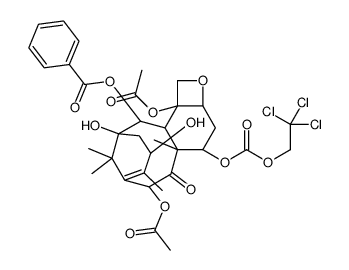
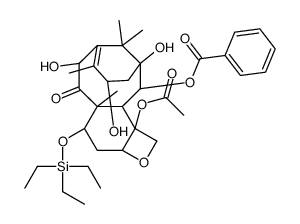
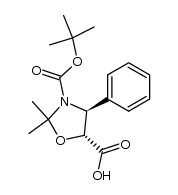
![13-{[(3-t-Boc)-2,2-dimethyl-4S-phenyl-1,3-oxazolidin-5R-yl]formyl}-7-O-(2,2,2-trichloroethyl)oxy]carbonyl) Baccatin III结构式](https://image.chemsrc.com/caspic/107/143527-73-5.png)


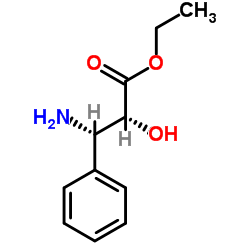
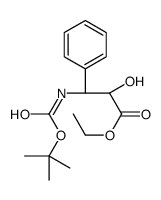
![3-(t-Boc)-2,2-dimethyl-4-phenyl-1,3-oxazolidin-5-yl]formic Acid Ethyl Ester结构式](https://image.chemsrc.com/caspic/069/143527-74-6.png)
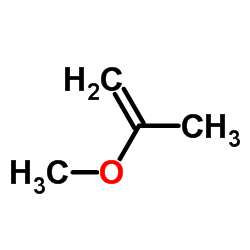
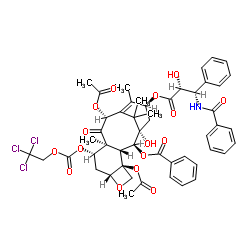
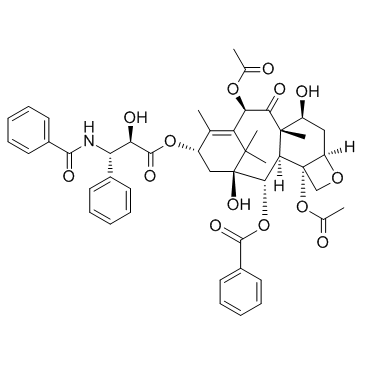
![N-Des-t-boc-10-去乙酰基7,10-O-双{[((2,2,2-三氯乙基)氧基]羰基}多西他赛结构式](https://image.chemsrc.com/caspic/353/114915-16-1.png)
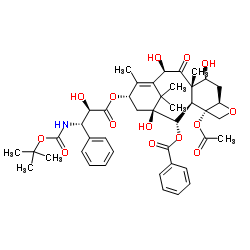
![13-{[(3-N-Boc)-2,2-dimethyl-4S-phenyl-1,3-oxazolidin-5R-yl]formyl}-10-deacetyl-7,10-bis{[(2,2,2-trichloroethyl)oxy]carbonyl} Baccatin III结构式](https://image.chemsrc.com/caspic/493/143527-76-8.png)