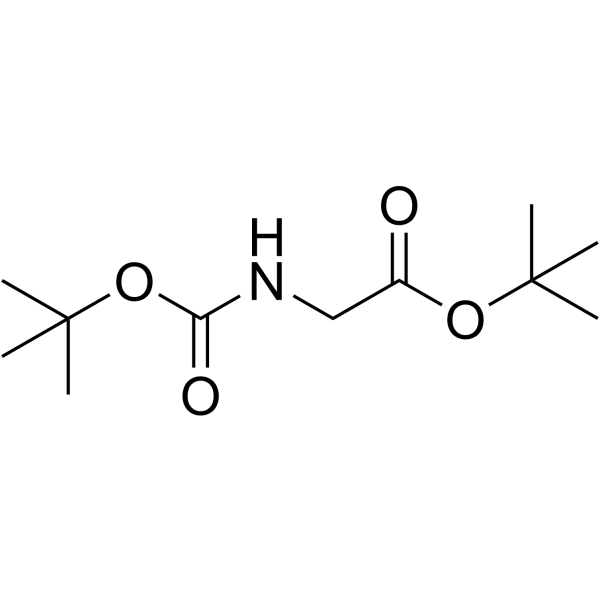
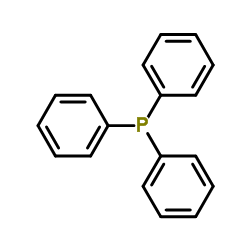
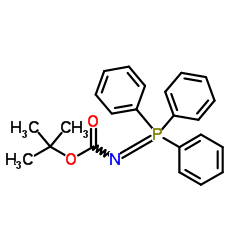
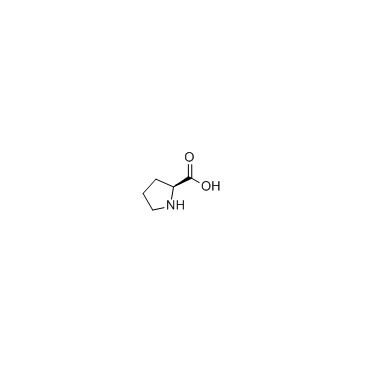
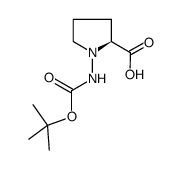
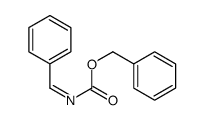
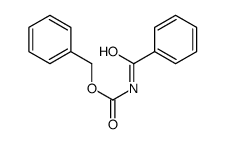
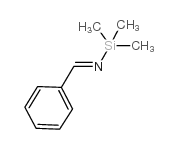
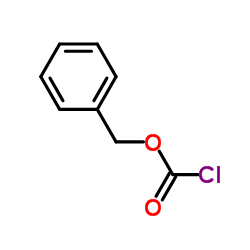
|
~% |
|
~% |
|
~% |
|
~90% |
|
~67% |
|
~70% |
|
~% |
|
~35% |
|
~%
详细
|
|
~92% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
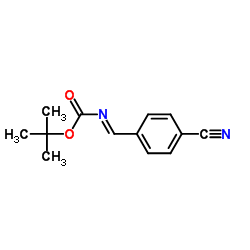
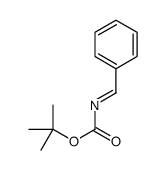
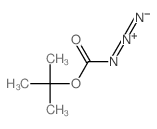
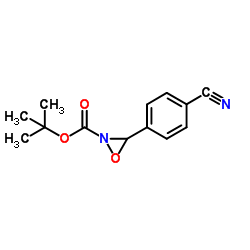
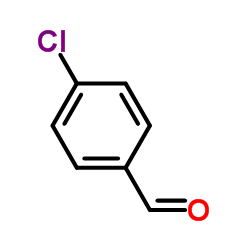


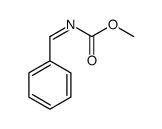

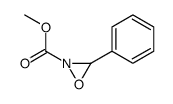
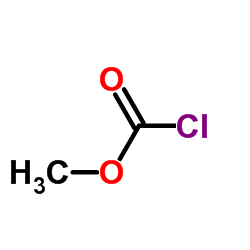
![methyl N-[(4-chlorophenyl)methylidene]carbamate结构式](https://image.chemsrc.com/caspic/370/199604-20-1.png)