|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~76% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~67% |

![2-甲酰基-4h,5h,6h,7h-噻吩并[3,2-c]吡啶-5-羧酸叔丁酯结构式](https://image.chemsrc.com/caspic/419/165947-55-7.png)
![5-Boc-4,5,6,7-四氢噻吩并[3,2-c]吡啶-2-羧酸乙酯结构式](https://image.chemsrc.com/caspic/344/623564-30-7.png)
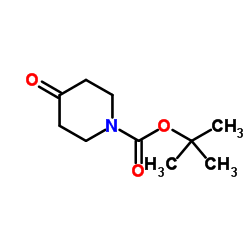

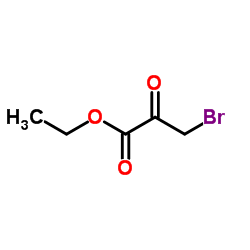
![7-甲基-5,6,7,8-四氢咪唑并[1,2-a]吡嗪-2-羧酸乙酯结构式](https://image.chemsrc.com/caspic/406/623564-19-2.png)
![咪唑并[1,2-a]吡嗪-2-甲酸乙酯结构式](https://image.chemsrc.com/caspic/483/77112-52-8.png)
![(6,7-二氢-5H-吡唑并[5,1-b][1,3]噁嗪-2-基)甲醇结构式](https://image.chemsrc.com/caspic/291/623565-62-8.png)
![6,7-二氢-5H-吡唑并[5,1-b][1,3]噁嗪-2-甲醛结构式](https://image.chemsrc.com/caspic/253/623565-63-9.png)
![4,5,6,7-四氢-[1,2,3]恶二唑并[3,4-a]吡啶-8-鎓-3-醇酸盐结构式](https://image.chemsrc.com/caspic/015/105786-95-6.png)
![(4,5,6,7-四氢吡唑并[1,5-a]吡啶-2-基)甲醇结构式](https://image.chemsrc.com/caspic/285/623564-49-8.png)
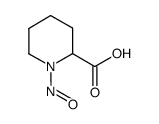
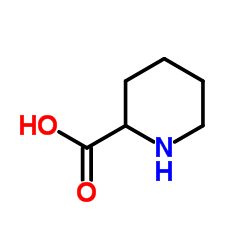
![4,5,6,7-四氢吡唑[1,5-a]吡啶-2-羧酸乙酯结构式](https://image.chemsrc.com/caspic/458/307307-84-2.png)
![4,5,6,7-四氢吡唑并[1,5-a]吡啶-2-甲醛结构式](https://image.chemsrc.com/caspic/127/307313-06-0.png)
![4H-吡唑[5,1-C][1,4] 6,7-二氢恶嗪-2-羧酸乙酯结构式](https://image.chemsrc.com/caspic/298/623565-57-1.png)
![(6,7-二氢-4H-吡唑并[5,1-c][1,4]噁嗪-2-基)甲醇结构式](https://image.chemsrc.com/caspic/260/623565-58-2.png)
![6,7-二氢-5H-吡唑并[5,1-b] [1,3]恶嗪-2-羧酸乙酯结构式](https://image.chemsrc.com/caspic/254/153597-59-2.png)