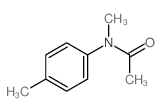
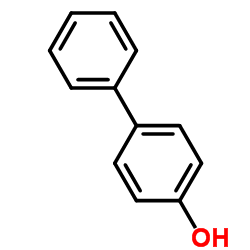
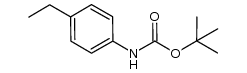
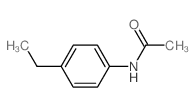
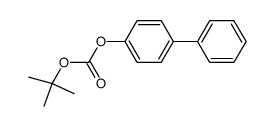
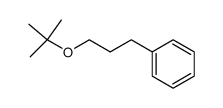
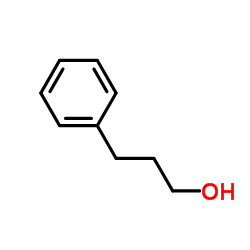
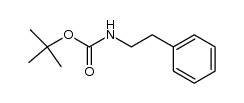
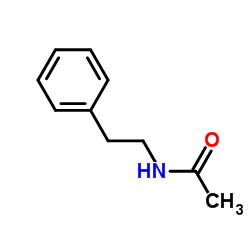
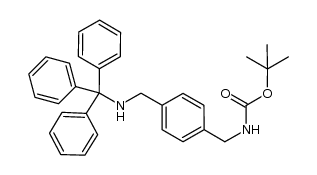
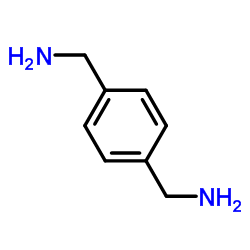
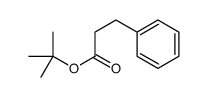
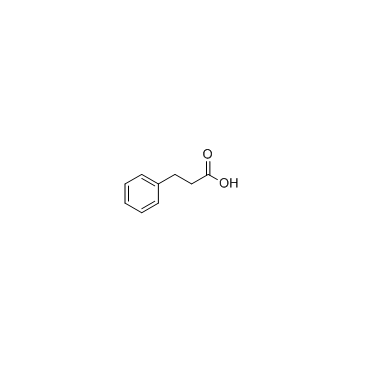
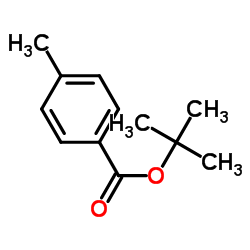
|
~82% |
|
~95% |
|
~73% |
|
~93% |
|
~71% |
|
~80% |
|
~99% |
|
~94% |
|
~94% |