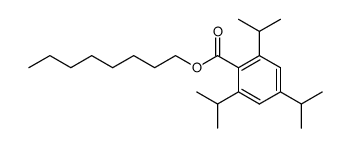
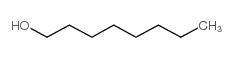
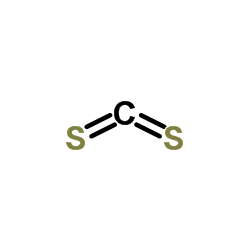
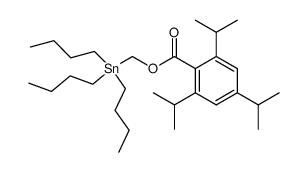
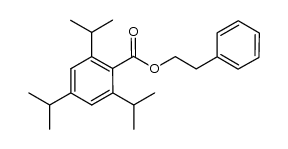
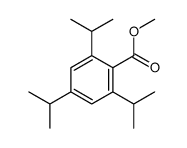
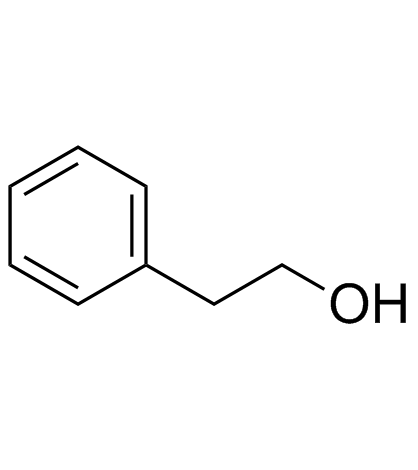
|
~99% |
|
~65% |
|
~15%
详细
|
|
~40% |
|
~93% |
|
~87% |
![S-methyl O-[1-phenyl-2-(2,4,6-triisopropylbenzoyloxy)ethyl] dithiocarbonate结构式](https://image.chemsrc.com/caspic/192/1133931-92-6.png)