Ristomycin sulfate
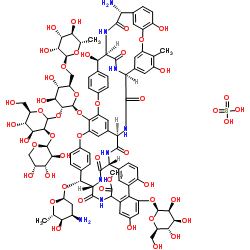
Ristomycin sulfate structure
|
Common Name | Ristomycin sulfate | ||
|---|---|---|---|---|
| CAS Number | 11140-99-1 | Molecular Weight | 2165.996 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C95H110N8O44.H2SO4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of Ristomycin sulfateRistomycin sulfate is a glycopeptide antibiotic isolated from Nocardia lurida[1]. |
| Name | ristocetin sulfate salt |
|---|---|
| Synonym | More Synonyms |
| Description | Ristomycin sulfate is a glycopeptide antibiotic isolated from Nocardia lurida[1]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C95H110N8O44.H2SO4 |
|---|---|
| Molecular Weight | 2165.996 |
| Exact Mass | 2164.628906 |
| PSA | 899.20000 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | 36/38 |
| Safety Phrases | 22-24/25-37/39-26 |
| RIDADR | UN 3249 |
| RTECS | VJ8650000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
|
Overproduction of Ristomycin A by activation of a silent gene cluster in Amycolatopsis japonicum MG417-CF17.
Antimicrob. Agents Chemother. 58(10) , 6185-96, (2014) The emergence of antibiotic-resistant pathogenic bacteria within the last decades is one reason for the urgent need for new antibacterial agents. A strategy to discover new anti-infective compounds is... |
|
|
Jordan, D.C., D. Gottlieb and P. Shaw,, ed.
Antibiotics New York , (1967) 1 , 84
|
|
|
Ristocetin- and thrombin-induced platelet aggregation at physiological shear rates: differential roles for GPIb and GPIIb-IIIa receptor.
Thromb. Haemost. 80 , 428-436, (1998) We recently reported that washed platelets (WP) activated with ADP and expressing surface-bound vWF aggregated in flow through small tubes or in a cylindrical couette device at physiological shear rat... |
| RISTOCETIN A SULFATE |
| Methyl (1S,2R,18R,19R,22R,34S,37R,40R)-22-amino-2-[(3-amino-2,3,6-trideoxy-α-L-ribo-hexopyranosyl)oxy]-64-{[β-D-arabinopyranosyl-(1->2)-D-mannopyranosyl-(1->2)-[6-deoxy-α-L-mannopyranosyl-( 1->6)]-β-D-glucopyranosyl]oxy}-18,26,31,44,49-pentahydroxy-47-(β-D-mannopyranosyloxy)-30-methyl-21,35,38,54,56,59-hexaoxo-7,13,28-trioxa-20,36,39,53,55,58-hexaazaundecacyclo[38.14.2.2.2.2.1.1.1.1~41, |
| Ristocetin sulfate salt |
| RISTOMYCIN MONOSULFATE |
| RISTOCETINSULFATQ |
| Ristocetin A sulphate |
| RISTOCETIN SULFATE |
| RISTOMYCIN |
| SPONTIN |
| ristomycin,sulfate |
| RISTON |