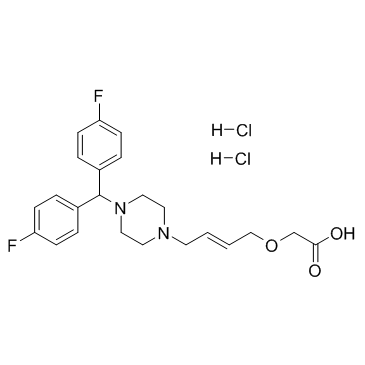
| Description |
SUN 1334H is a potent, orally active, highly selective H1 receptor antagonist, with Ki of 9.7 nM.
|
| Related Catalog |
|
| Target |
Ki: 9.7 nM (H1 receptor)[1]
|
| In Vitro |
SUN-1334H causes potent inhibition of histamine induced contractions of isolated guinea-pig ileum with an IC50 (half the maximal inhibitory concentration) of 0.198 μM. In CHO-K1/hERG cells, SUN-1334H does not modulate hERG K+-currents at concentrations as high as 100 μM[1]. SUN-1334H, cetirizine and hydroxyzine cause comparable inhibition of NLF leukocytes, IL-4 and total protein concentrations[2].
|
| In Vivo |
SUN-1334H potently inhibits histamine-induced bronchospasm over 24 hours following oral administration and completely suppresses histamine-induced skin wheal in beagle dogs and ovalbumin-induced rhinitis in guinea pigs[1]. In skin allergy models, SUN-1334H shows potent reduction of passive and active cutaneous anaphylactic reactions. In central nervous system side effects models, SUN-1334H, desloratadine and fexofenadine are devoid of any significant effects[2].
|
| References |
[1]. Mandhane SN, et al. Preclinical efficacy and safety pharmacology of SUN-1334H, a potent orally active antihistamine agent. Drugs R D. 2008;9(2):93-112. [2]. Mandhane SN, et al. Characterization of anti-inflammatory properties and evidence for no sedation liability for the novel antihistamine SUN-1334H. Int Arch Allergy Immunol. 2010;151(1):56-69.
|