PLX-4720
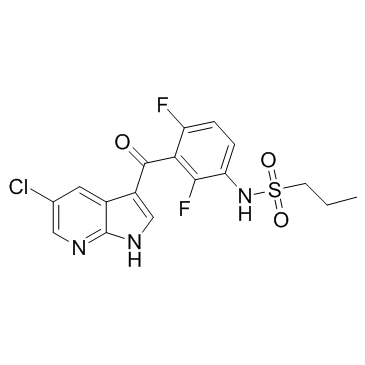
PLX-4720 structure
|
Common Name | PLX-4720 | ||
|---|---|---|---|---|
| CAS Number | 918505-84-7 | Molecular Weight | 413.826 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 621.4±65.0 °C at 760 mmHg | |
| Molecular Formula | C17H14ClF2N3O3S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 329.6±34.3 °C | |
Use of PLX-4720PLX-4720 is a potent and selective inhibitor of B-RafV600E with IC50 of 13 nM in a cell-free assay, equally potent to c-Raf-1(Y340D and Y341D mutations), and 10-fold selectivity for B-RafV600E than wild-type B-Raf. |
| Name | N-[3-(5-chloro-1H-pyrrolo[2,3-b]pyridine-3-carbonyl)-2,4-difluorophenyl]propane-1-sulfonamide |
|---|---|
| Synonym | More Synonyms |
| Description | PLX-4720 is a potent and selective inhibitor of B-RafV600E with IC50 of 13 nM in a cell-free assay, equally potent to c-Raf-1(Y340D and Y341D mutations), and 10-fold selectivity for B-RafV600E than wild-type B-Raf. |
|---|---|
| Related Catalog | |
| Target |
B-RafV600E:13 nM (IC50) B-Raf:160 nM (IC50) BRK:130 nM (IC50) FRK:1300 nM (IC50) Csk:1500 nM (IC50) Src:1700 nM (IC50) FAK:1700 nM (IC50) FGFR:1900 nM (IC50) KDR:2300 nM (IC50) HGK:2800 nM (IC50) CSF1R:3300 nM (IC50) Aurora A:3400 nM (IC50) |
| In Vitro | PLX-4720 displays >10 times selectivity against wild type B-Raf, and >100 times selectivity over other kinases such as Frk, Src, Fak, FGFR, and Aurora A with IC50 of 1.3-3.4 μM. PLX-4720 significantly inhibits the ERK phosphorylation in cell lines bearing B-RafV600E with IC50 of 14-46 nM, but not the cells with wild-type B-Raf. PLX-4720 significantly inhibits the growth of tumor cell lines bearing the B-RafV600E oncogene, such as COLO205, A375, WM2664, and COLO829 with GI50 of 0.31 μM, 0.50 μM, 1.5 μM, and 1.7 μM, respectively. In addition, PLX-4720 treatment at 1 μM induces cell cycle arrest and apoptosis exclusively in the B-RafV600E-positive 1205Lu cells, but not in the B-Raf wild-type C8161 cells[1]. PLX-4720 treatment (10 μM) significantly induces > 14-fold expression of BIM in the PTEN+ cells, compared with the PTEN- cell lines (4-fold), giving an explanation of the resistance of PTEN- cells to PLX-4720-induced apoptosis[2]. |
| In Vivo | Oral administration of PLX-4720 at 20 mg/kg/day induces significant tumor growth delays and regressions in B-RafV600E-dependent COLO205 tumor xenografts, without obvious adverse effects in mice even at dose of 1 g/kg. PLX-4720 at 100 mg/kg twice daily almost completely eliminates the 1205Lu xenografts bearing B-RafV600E, while has no activity against C8161 xenografts bearing wild-type B-Raf. The anti-tumor effects of PLX-4720 correlate with the blockade of MAPK pathway in those cells harboring the V600E mutation[1]. PLX-4720 treatment at 30 mg/kg/day significant inhibits the tumor growth of 8505c xenografts by >90%, and dramatically decreases distant lung metastases[3]. |
| Kinase Assay | For each enzyme (0.1 ng), 20-μL reactions are carried out in 20 mM Hepes (pH 7.0), 10 mM MgCl2, 1 mM DTT, 0.01% Tween-20, 100 nM biotin-MEK protein, various ATP concentrations, and increasing concentrations of PLX-4720 at room temperature. Reactions are stopped at 2, 5, 8, 10, 20, and 30 minutes with 5 μL of a solution containing 20 mM Hepes (pH 7.0), 200 mM NaCl, 80 mM EDTA, and 0.3% BSA. The stop solution also includes phospho-MEK Antibody, Streptavidin-coated Donor beads and Protein A Acceptor beads from the AlphaScreen Protein A Detection Kit. The antibody and beads are preincubated in stop solution in the dark at room temperature for 30 minutes. The final dilution of antibody is 1/2,000, and the final concentration of each bead is 10 μg/mL. The assay plates are incubated at room temperature for one hour then are read on a PerkinElmer AlphaQuest reader. |
| Cell Assay | Cells are treated with various concentrations PLX-4720 for 24, 48, and 72 hours. Cell proliferation is measured by using the CellTiter-Glo Luminescent Cell Viability Assay or MTT assay. For cell cycle analysis, supernatant and cells are collected, pelleted, and fixed with 70% ethanol. Before staining with propidium iodide (10 μg/mL), cells are incubated for 1 hour at 37°C in 0.5 mg/mL RNase I to rid samples of residual RNA contamination. Samples are then analyzed by using the EPICS XL apparatus. For the assessment of apoptosis, media and cells are harvested and pelleted before staining with annexin-FITC and propidium iodide. Samples are subsequently analyzed by using the EPICS XL apparatus. |
| Animal Admin | Metastatic melanoma cells (2×106) are s.c. injected into the flanks of SCID mice and allowed appr 2 weeks to reach 0.125 mm3 in volume. Subsequently, the animals receive either 100 mg/kg PLX4720 (oral gavage) or vehicle control twice daily for 15 days. Tumor volume is recorded every 72 h. The average tumor size for each respective group is normalized to the tumor volume at the first day of treatment. After 15 days of treatment, animals are killed and tumors are excised, fixed in formalin, paraffin-embedded, and analyzed by immunohistochemistry. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 621.4±65.0 °C at 760 mmHg |
| Molecular Formula | C17H14ClF2N3O3S |
| Molecular Weight | 413.826 |
| Flash Point | 329.6±34.3 °C |
| Exact Mass | 413.041260 |
| PSA | 100.30000 |
| LogP | 3.14 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.646 |
| InChIKey | YZDJQTHVDDOVHR-UHFFFAOYSA-N |
| SMILES | CCCS(=O)(=O)Nc1ccc(F)c(C(=O)c2c[nH]c3ncc(Cl)cc23)c1F |
| Storage condition | -20°C |
| PLX 4720,PLX-4720 |
| N-{3-[(5-Chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)carbonyl]-2,4-difluorophenyl}-1-propanesulfonamide |
| PLX-4720 |
| 1-Propanesulfonamide, N-[3-[(5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)carbonyl]-2,4-difluorophenyl]- |
| S1152_Selleck |
| PLX4720 |