(2R,3R,4R,5R)-5-(6-amino-1-methyl-2H-purin-9-yl)-2-(hydroxymethyl)-4-m ethoxy-oxolan-3-ol
Modify Date: 2025-08-25 17:49:37
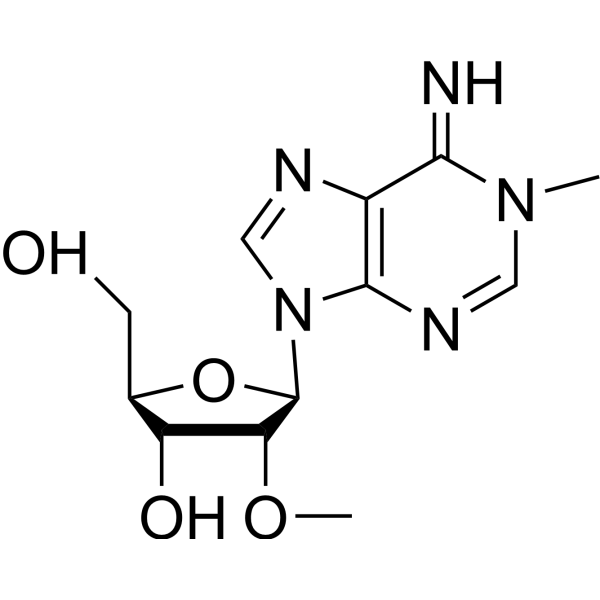
(2R,3R,4R,5R)-5-(6-amino-1-methyl-2H-purin-9-yl)-2-(hydroxymethyl)-4-m ethoxy-oxolan-3-ol structure
|
Common Name | (2R,3R,4R,5R)-5-(6-amino-1-methyl-2H-purin-9-yl)-2-(hydroxymethyl)-4-m ethoxy-oxolan-3-ol | ||
|---|---|---|---|---|
| CAS Number | 91101-00-7 | Molecular Weight | 295.29 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C12H17N5O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of (2R,3R,4R,5R)-5-(6-amino-1-methyl-2H-purin-9-yl)-2-(hydroxymethyl)-4-m ethoxy-oxolan-3-ol2’-O-Methyl-N1-methyladenosine is a purine nucleoside analog. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc[1]. |
| Name | (2R,3R,4R,5R)-5-(6-amino-1-methyl-2H-purin-9-yl)-2-(hydroxymethyl)-4-methoxyoxolan-3-ol |
|---|---|
| Synonym | More Synonyms |
| Description | 2’-O-Methyl-N1-methyladenosine is a purine nucleoside analog. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc[1]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C12H17N5O4 |
|---|---|
| Molecular Weight | 295.29 |
| Exact Mass | 297.14400 |
| PSA | 118.36000 |
| 2'-O-Methyl-1-methyladenosine |