Myclobutanil
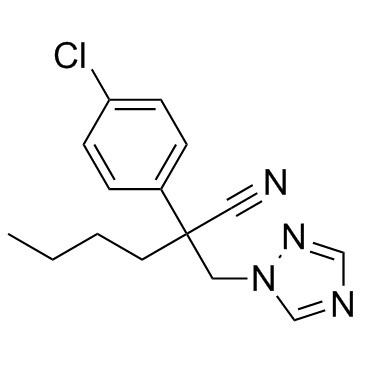
Myclobutanil structure
|
Common Name | Myclobutanil | ||
|---|---|---|---|---|
| CAS Number | 88671-89-0 | Molecular Weight | 288.775 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 465.2±55.0 °C at 760 mmHg | |
| Molecular Formula | C15H17ClN4 | Melting Point | 63-68°C | |
| MSDS | Chinese USA | Flash Point | 235.2±31.5 °C | |
| Symbol |


GHS07, GHS08, GHS09 |
Signal Word | Warning | |
Use of MyclobutanilMyclobutanil is a conazole class fungicide widely used as an agrichemical. |
| Name | myclobutanil |
|---|---|
| Synonym | More Synonyms |
| Description | Myclobutanil is a conazole class fungicide widely used as an agrichemical. |
|---|---|
| Related Catalog | |
| In Vitro | Myclobutanil reduces cell viability to <50% at 100 ppm and to <10% at 500 ppm. Myclobutanil promotes a slight, but significant, increase in fatty acid (FA)-induced steatotosis at doses from 1 to 100 ppm. Anti-apoptotic biomarkers are significantly reduced by Myclobutanil[1]. |
| Kinase Assay | To further evaluate apoptosis, cell extracts are collected after 24 h of exposure to Myclobutanil, centrifuged, and analyzed with a multiplex biometric ELISA-based immunoassay containing dyed microspheres conjugated to a monoclonal antibody specific for the target protein. Apoptosis biomarkers are BCL-xL/Bak dimer and Mcl-1/Bak dimer, quantified using RBM Apoptosis Panel 3. Each experiment is performed in triplicate and apoptosis biomarker levels determined using the Bio-Plex Array Reader. The analytic concentrations are calculated using a standard curve, according to the manufacturer’s instructions[1]. |
| Cell Assay | The hepatoma cell line HepG2 is used in this study. The cells are grown on tissue culture plates in an incubator with a humidified atmosphere (95% air/5% CO2 v/v) at 37°C. Steatosis is induced by incubating the hepatocytes with 6 mM of a 1:1 v/v mixture of oleic (18:1) and linoleic (18:2) fatty acids (Fas) for 24 h. After a wash with PBS, cells are exposed for an additional 24 h to Myclobutanil at 0.1, 1, 10, 100 or 500 ppm. Cytotoxicity is assessed in HepG2 cells (1.0×105 cells/well in 24-well plates) by measuring the reduction of the tetrazolium dye 3-(4, 5-dimethylthiazol-2-yl)-5-(3carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTT)[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 465.2±55.0 °C at 760 mmHg |
| Melting Point | 63-68°C |
| Molecular Formula | C15H17ClN4 |
| Molecular Weight | 288.775 |
| Flash Point | 235.2±31.5 °C |
| Exact Mass | 288.114166 |
| PSA | 54.50000 |
| LogP | 2.82 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.589 |
| Storage condition | 0-6°C |
| Water Solubility | 142 mg/L (25 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS07, GHS08, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H319-H361d-H411 |
| Precautionary Statements | P273-P281-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36;R51/53;R63 |
| Safety Phrases | S36/37-S46-S61 |
| RIDADR | UN 3077 |
| RTECS | XZ5257000 |
| HS Code | 2933990015 |
| HS Code | 2933990015 |
|---|---|
| Summary | 2933990015 3-((1h-1,2,4-triazol-1-yl)methyl)-1-(4-chlorophenyl)-4,4-dimethylpentan-3-ol。supervision conditions:s(import or export registration certificate for pesticides)。VAT:17.0%。tax rebate rate:9.0%。MFN tarrif:6.5%。general tariff:20.0% |
|
The effects of the fungicides fenhexamid and myclobutanil on SH-SY5Y and U-251 MG human cell lines.
Environ. Toxicol. Pharmacol. 38(3) , 968-76, (2014) Mixtures of pesticides in foodstuffs and the environment are ubiquitous in the developed world and although agents are usually exhaustively tested individually, the toxicological implications of pesti... |
|
|
Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus.
PLoS ONE 7(3) , e31801, (2012) Azoles play an important role in the management of Aspergillus diseases. Azole resistance is an emerging global problem in Aspergillus fumigatus, and may develop through patient therapy. In addition, ... |
|
|
Acaricide, fungicide and drug interactions in honey bees (Apis mellifera).
PLoS ONE 8(1) , e54092, (2013) Chemical analysis shows that honey bees (Apis mellifera) and hive products contain many pesticides derived from various sources. The most abundant pesticides are acaricides applied by beekeepers to co... |
| 1H-1,2,4-Triazole-1-propanenitrile, α-butyl-α-(4-chlorophenyl)- |
| α-Butyl-α-(4-chlorophenyl)-1H-1,2,4-triazole-1-propanenitrile |
| Systhane Technical |
| rac-(2R)-2-(4-chlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)hexanenitrile |
| 2-(4-Chlorphenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)hexanonitril |
| Synthane 12E |
| 2-(4-Chlorophényl)-2-(1H-1,2,4-triazol-1-ylméthyl)hexanenitrile |
| Nova W |
| 2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile |
| 2-(4-Chlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)hexanenitrile |
| Systhane |
| Systhane 6 Flo |
| a-Butyl-a-(4-chlorophenyl)-1H-1,2,4-triazole-1-propanenitrile |
| Rally |
| (RS)-2-(4-chlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)hexanenitrile |
| MFCD00144818 |
| T5NN DNJ A1X4&CN&R DG |
| Myclobutanil |

