Pipernonaline
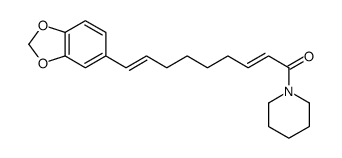
Pipernonaline structure
|
Common Name | Pipernonaline | ||
|---|---|---|---|---|
| CAS Number | 88660-10-0 | Molecular Weight | 341.44400 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C21H27NO3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of PipernonalinePipernonaline is a piperine derivative with antiprostate cancer activity. Pipernonaline inhibits the proliferation of androgen-dependent/independent LNCaP/PC-3 prostate cells. Pipernonaline activates caspase-3 and promotes procaspase-3/PARP cleavage. Pipernonaline also mediates reactive oxygen species (ROS) production, increased intracellular Ca(2+), and mitochondrial membrane depolarization[1]. |
| Name | 9-(1,3-benzodioxol-5-yl)-1-piperidin-1-ylnona-2,8-dien-1-one |
|---|---|
| Synonym | More Synonyms |
| Description | Pipernonaline is a piperine derivative with antiprostate cancer activity. Pipernonaline inhibits the proliferation of androgen-dependent/independent LNCaP/PC-3 prostate cells. Pipernonaline activates caspase-3 and promotes procaspase-3/PARP cleavage. Pipernonaline also mediates reactive oxygen species (ROS) production, increased intracellular Ca(2+), and mitochondrial membrane depolarization[1]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C21H27NO3 |
|---|---|
| Molecular Weight | 341.44400 |
| Exact Mass | 341.19900 |
| PSA | 38.77000 |
| LogP | 4.49550 |
| InChIKey | PKLGRWSJBLGIBF-JMQWPVDRSA-N |
| SMILES | O=C(C=CCCCCC=Cc1ccc2c(c1)OCO2)N1CCCCC1 |
|
Antihyperlipidemic compounds from the fruit of Piper longum L.
Phytother Res. 23(8) , 1194-6, (2009) A bioassay-guided isolation of an ethanol extract of the fruit of Piper longum L. yielded piperlonguminine, piperine and pipernonaline, as the main antihyperlipidemic constituents. They exhibit apprec... |
|
|
Alkamides from the fruits ofPiper longumandPiper nigrumdisplaying potent cell adhesion inhibition
Bioorg. Med. Chem. Lett. 18 , 4544-6, (2008) Eight alkamides 1– 8 were isolated from EtOH extracts of the fruits of Piper longum and nigrum. Among the tested alkamide derivatives 1– 8, dehydropipernonaline (8) showed the most inhibition of the d... |
| Piperidine,1-[(2E,8E)-9-(1,3-benzodioxol-5-yl)-1-oxo-2,8-nonadienyl] |