Phthalazinone pyrazole
Modify Date: 2024-01-08 18:41:42
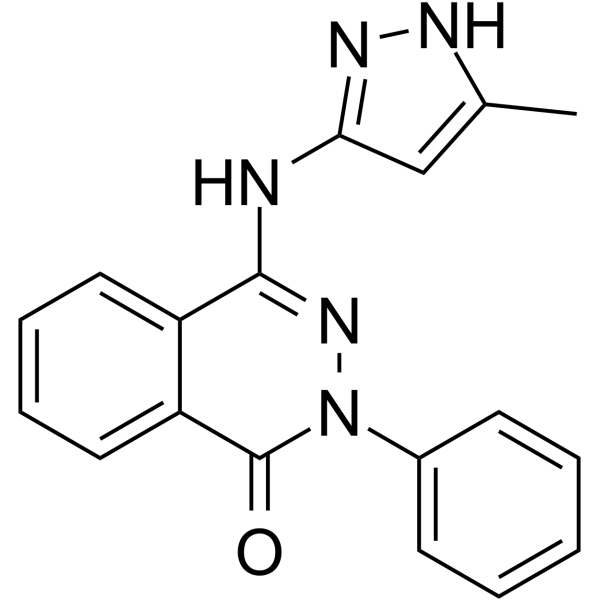
Phthalazinone pyrazole structure
|
Common Name | Phthalazinone pyrazole | ||
|---|---|---|---|---|
| CAS Number | 880487-62-7 | Molecular Weight | 317.345 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 557.7±52.0 °C at 760 mmHg | |
| Molecular Formula | C18H15N5O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 291.1±30.7 °C | |
Use of Phthalazinone pyrazolePhthalazinone pyrazole is a potent, selective, and orally active inhibitor of Aurora-A kinase with an IC50 of 0.031 μM. Phthalazinone pyrazole can arrests mitosis and subsequently inhibit tumor growth via apoptosis of proliferating cells. Phthalazinone pyrazole suppresses the epithelial-mesenchymal transition (EMT) during the differentiation of hepatocyte-like cells (HLCs) from human embryonic stem cells[1][2]. |
| Name | 4-[(5-Methyl-1H-pyrazol-3-yl)amino]-2-phenyl-1(2H)-phthalazinone |
|---|---|
| Synonym | More Synonyms |
| Description | Phthalazinone pyrazole is a potent, selective, and orally active inhibitor of Aurora-A kinase with an IC50 of 0.031 μM. Phthalazinone pyrazole can arrests mitosis and subsequently inhibit tumor growth via apoptosis of proliferating cells. Phthalazinone pyrazole suppresses the epithelial-mesenchymal transition (EMT) during the differentiation of hepatocyte-like cells (HLCs) from human embryonic stem cells[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Aurora-A:0.031 μM (IC50) |
| In Vitro | Phthalazinone pyrazole (1 and 10 μM; 30 hours) enhances the proliferative capacity of HLCs[2]. Phthalazinone pyrazole (1, 10, and 100 μM; 5 days) enhances hepatic morphological changes in differentiated HLCs without cytotoxicity[2]. Phthalazinone pyrazole (1 and 10 μM; 5 and 17 days) suppresses the EMT and induced maturation of HLCs through the inhibition of the AKT signaling pathway by the off target effect with concomitant upregulation of HNF4α rather than direct inhibition of Aurora-A. The result is confirmed by western blot and qPCR[2]. Cell Proliferation Assay[2] Cell Line: Hepatocyte-like cells (HLCs) Concentration: 1 and 10 μM Incubation Time: 30 hours Result: Enhanced the proliferative capacity of HLCs. Cell Cytotoxicity Assay[2] Cell Line: ES-HLCs, iPS-HLCs, Huh7 cells Concentration: 1, 10, and 100 μM Incubation Time: 5 days Result: Showed no cytotoxic effects on HLCs. Western Blot Analysis[2] Cell Line: HLCs Concentration: 1 and 10 μM Incubation Time: 5 and 17 days Result: Markedly inhibited the phosphorylation of AKT and activated GSK-3β, which in turn inhibited Snail expression and increased HNF4α. Phthalazinone pyrazole didn’t significantly reduce the phosphorylation of Aurora-A. RT-PCR[2] Cell Line: HLCs Concentration: 1 and 10 μM Incubation Time: 5 and 17 days Result: Markedly inhibited the phosphorylation of AKT mRNA and activated GSK-3β mRNA, which in turn inhibited Snail mRNA expression and increased HNF4α mRNA. Phthalazinone pyrazole didn’t significantly reduce the phosphorylation of Aurora-A mRNA. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 557.7±52.0 °C at 760 mmHg |
| Molecular Formula | C18H15N5O |
| Molecular Weight | 317.345 |
| Flash Point | 291.1±30.7 °C |
| Exact Mass | 317.127655 |
| PSA | 78.83000 |
| LogP | 3.03 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.716 |
| 4-[(5-Methyl-1H-pyrazol-3-yl)amino]-2-phenyl-1(2H)-phthalazinone |
| 1(2H)-Phthalazinone, 4-[(5-methyl-1H-pyrazol-3-yl)amino]-2-phenyl- |
| Phthalazinone Pyrazole |