Sodium formaldehyde bisulfite
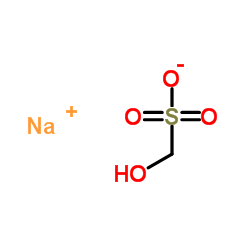
Sodium formaldehyde bisulfite structure
|
Common Name | Sodium formaldehyde bisulfite | ||
|---|---|---|---|---|
| CAS Number | 870-72-4 | Molecular Weight | 134.087 | |
| Density | N/A | Boiling Point | 184ºC | |
| Molecular Formula | CH3NaO4S | Melting Point | 200 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 184 °C | |
| Name | Formaldehyde Sodium Bisulfite |
|---|---|
| Synonym | More Synonyms |
| Boiling Point | 184ºC |
|---|---|
| Melting Point | 200 °C (dec.)(lit.) |
| Molecular Formula | CH3NaO4S |
| Molecular Weight | 134.087 |
| Flash Point | 184 °C |
| Exact Mass | 133.964981 |
| PSA | 85.81000 |
| InChIKey | UOULCEYHQNCFFH-UHFFFAOYSA-M |
| SMILES | O=S(=O)([O-])CO.[Na+] |
| Storage condition | -20°C |
| Water Solubility | 800 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
Sodium formalde... CAS#:870-72-4 |
| Literature: Tetrahedron, , vol. 40, # 10 p. 1855 - 1856 |
|
~% 
Sodium formalde... CAS#:870-72-4 |
| Literature: Journal of the Chemical Society. Perkin Transactions 2, , # 5 p. 941 - 946 |
|
~% 
Sodium formalde... CAS#:870-72-4 |
| Literature: Phosphorus and Sulfur and the Related Elements, , vol. 11, p. 295 - 302 |
|
~% 
Sodium formalde... CAS#:870-72-4 |
| Literature: Phosphorus and Sulfur and the Related Elements, , vol. 11, p. 295 - 302 |
|
~% 
Sodium formalde... CAS#:870-72-4 |
| Literature: Phosphorus and Sulfur and the Related Elements, , vol. 11, p. 295 - 302 |
|
~% 
Sodium formalde... CAS#:870-72-4 |
| Literature: Phosphorus and Sulfur and the Related Elements, , vol. 11, p. 295 - 302 |
|
~% 
Sodium formalde... CAS#:870-72-4 |
| Literature: Phosphorus and Sulfur and the Related Elements, , vol. 11, p. 295 - 302 |
|
~% 
Sodium formalde... CAS#:870-72-4 |
| Literature: Phosphorus and Sulfur and the Related Elements, , vol. 11, p. 295 - 302 |
| Precursor 6 | |
|---|---|
| DownStream 10 | |
| HS Code | 2942000000 |
|---|
|
Improved production of 3-hydroxypropionaldehyde by complex formation with bisulfite during biotransformation of glycerol.
Biotechnol. Bioeng. 110(4) , 1243-8, (2013) 3-Hydroxypropionaldehyde (3HPA) is an important specialty chemical which can be produced from glycerol using resting cells of Lactobacillus reuteri. This biocatalytic route, however, suffers from subs... |
|
|
Determination of free and reversibly-bound sulfite in selected foods by high-performance liquid chromatography with fluorometric detection.
J. AOAC Int. 91(1) , 98-102, (2008) A rapid and accurate method for measuring low part-per-million levels of free and reversibly-bound sulfites in selected foods by using high-performance liquid chromatography (HPLC) with fluorometric d... |
|
|
Light-induced formation of hydroxyl radicals in fog waters determined by an authentic fog constituent, hydroxymethanesulfonate.
Chemosphere 51(3) , 175-9, (2003) The determination of the photo-production rate of hydroxyl radical (OH) in atmospheric liquids is of fundamental importance to an understanding of atmospheric aquatic chemistry. Recently, several stud... |
| Formaldehyde-sodium bisulfite adduct |
| MFCD00044664 |
| Sodium formaldehyde bisulfite |
| Methanesulfonic acid, 1-hydroxy-, sodium salt (1:1) |
| Sodium hydroxymethanesulfonate |
| EINECS 212-800-9 |
| Hydroxymethanesulfonate, sodium salt |
| PS |
| PN |
| Hydroxymethanesulfonic acid monosodium salt |
| Sodium hydroxymethanesulphonate |


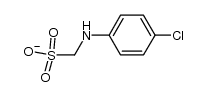



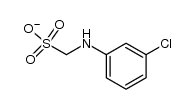
 CAS#:926-64-7
CAS#:926-64-7![[(4-methylphenyl)amino]methanesulfonic acid structure](https://image.chemsrc.com/caspic/220/28141-40-4.png) CAS#:28141-40-4
CAS#:28141-40-4 CAS#:13046-10-1
CAS#:13046-10-1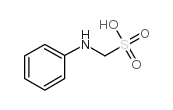 CAS#:103-06-0
CAS#:103-06-0![Acetonitrile,2-[(4-methylphenyl)amino]- structure](https://image.chemsrc.com/caspic/472/16728-84-0.png) CAS#:16728-84-0
CAS#:16728-84-0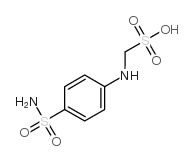 CAS#:122-89-4
CAS#:122-89-4![Methanesulfonic acid,1-[(3-chlorophenyl)amino]-, sodium salt (1:1) structure](https://image.chemsrc.com/caspic/030/28141-45-9.png) CAS#:28141-45-9
CAS#:28141-45-9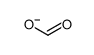 CAS#:71-47-6
CAS#:71-47-6![3-[6-(1,3-thiazolidin-3-yl)hexyl]-1,3-thiazolidine structure](https://image.chemsrc.com/caspic/326/88346-62-7.png) CAS#:88346-62-7
CAS#:88346-62-7
