CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
CX7065000
-
CHEMICAL NAME :
-
1H-1-Benzazepine-1-acetic acid, 2,3,4,5-tetrahydro-3-((1-(ethoxycarbonyl)-3-phenylpro pyl) amino)-2-oxo-, monohydrochloride, (S-(R*,R*))-
-
CAS REGISTRY NUMBER :
-
86541-74-4
-
LAST UPDATED :
-
199512
-
DATA ITEMS CITED :
-
8
-
MOLECULAR FORMULA :
-
C24-H28-N2-O5.Cl-H
-
MOLECULAR WEIGHT :
-
461.00
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CDREEA Cardiovascular Drug Reviews. (Raven Press, 1185 Avenue of the Americas, New York, NY 10036) V.6- 1988- Volume(issue)/page/year: 8,89,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
4019 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CDREEA Cardiovascular Drug Reviews. (Raven Press, 1185 Avenue of the Americas, New York, NY 10036) V.6- 1988- Volume(issue)/page/year: 8,89,1990
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
1 gm/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Olfaction) - effect, not otherwise specified Behavioral - food intake (animal) Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
CDREEA Cardiovascular Drug Reviews. (Raven Press, 1185 Avenue of the Americas, New York, NY 10036) V.6- 1988- Volume(issue)/page/year: 8,89,1990 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3650 mg/kg/1Y-C
-
TOXIC EFFECTS :
-
Behavioral - food intake (animal) Liver - changes in liver weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 19,3863,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
54750 mg/kg/1Y-C
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes primarily in glomeruli Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Blood - changes in spleen
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 19,3893,1991 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
81 gm/kg
-
SEX/DURATION :
-
male 60 day(s) pre-mating female 2 week(s) pre-mating - 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 19,3445,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
26 gm/kg
-
SEX/DURATION :
-
female 17-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain) Reproductive - Effects on Newborn - delayed effects
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 19,3471,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
11 gm/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - urogenital system Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 19,3453,1991
|
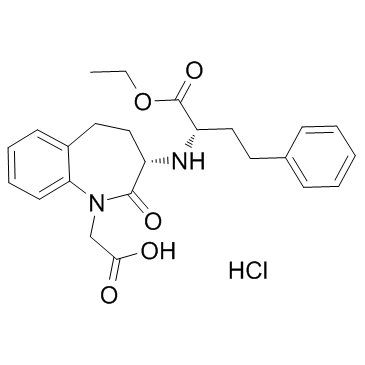
![(2S,3'S)-2-(1-tert-butoxycarbonylmethyl-2-oxo-2,3,4,5-tetrahydro-1H-benzo[b]azepin-3-ylamino)-4-phenylbutyric acid ethyl ester Structure](https://image.chemsrc.com/caspic/207/109010-61-9.png) CAS#:109010-61-9
CAS#:109010-61-9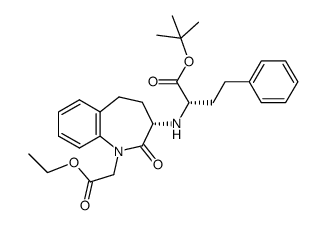 CAS#:859635-53-3
CAS#:859635-53-3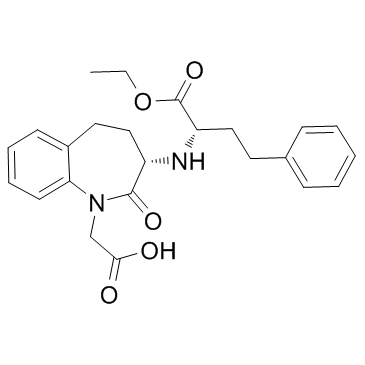 CAS#:86541-75-5
CAS#:86541-75-5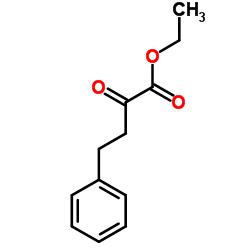 CAS#:64920-29-2
CAS#:64920-29-2 CAS#:86499-53-8
CAS#:86499-53-8![4,5-Dihydro-1H-benzo[b]azepin-2(3H)-one Structure](https://image.chemsrc.com/caspic/098/4424-80-0.png) CAS#:4424-80-0
CAS#:4424-80-0 CAS#:86499-22-1
CAS#:86499-22-1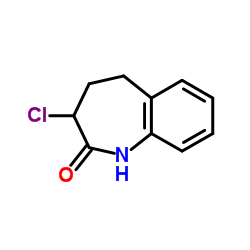 CAS#:86499-23-2
CAS#:86499-23-2