5(S),15(S)-DIHETE
Modify Date: 2025-08-25 12:04:54

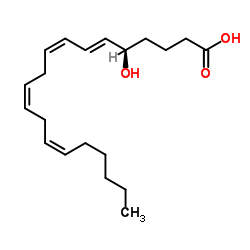
5(S),15(S)-DIHETE structure
|
Common Name | 5(S),15(S)-DIHETE | ||
|---|---|---|---|---|
| CAS Number | 82200-87-1 | Molecular Weight | 336.466 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 537.6±50.0 °C at 760 mmHg | |
| Molecular Formula | C20H32O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 293.0±26.6 °C | |
Use of 5(S),15(S)-DIHETE5(S)15(S)-DiHETE is an “activated” intermediate, inhibits platelet aggregation with an IC50 of 1.3 μM. 5(S)15(S)-DiHETE enhances the rate of either LXA4 or LXB4 biosynthesis[1]. |
| Name | 5(s), 15(s)-dihete |
|---|---|
| Synonym | More Synonyms |
| Description | 5(S)15(S)-DiHETE is an “activated” intermediate, inhibits platelet aggregation with an IC50 of 1.3 μM. 5(S)15(S)-DiHETE enhances the rate of either LXA4 or LXB4 biosynthesis[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 537.6±50.0 °C at 760 mmHg |
| Molecular Formula | C20H32O4 |
| Molecular Weight | 336.466 |
| Flash Point | 293.0±26.6 °C |
| Exact Mass | 336.230072 |
| PSA | 77.76000 |
| LogP | 3.87 |
| Vapour Pressure | 0.0±3.2 mmHg at 25°C |
| Index of Refraction | 1.527 |
|
~% 
5(S),15(S)-DIHETE CAS#:82200-87-1 |
| Literature: Mulugeta, Surafel; Suzuki, Takashi; Hernandez, Noemi Tejera; Griesser, Markus; Boeglin, William E.; Schneider, Claus Journal of Lipid Research, 2010 , vol. 51, # 3 p. 575 - 585 |
|
~% 
5(S),15(S)-DIHETE CAS#:82200-87-1 |
| Literature: Corey, E. J.; Su, Wei-guo; Cleaver, Martin B. Tetrahedron Letters, 1989 , vol. 30, # 32 p. 4181 - 4184 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| 5S,15S-DiHETE |
| (5S,6E,8Z,11Z,13E,15S)-5,15-Dihydroxy-6,8,11,13-icosatetraenoic acid |
| 5(S),15(S)-DIHETE |
| 5,15-dihydroxy-6,8,11,13-eicosatetraenoicacid |
| 6,8,11,13-Eicosatetraenoic acid, 5,15-dihydroxy-, (5S,6E,8Z,11Z,13E,15S)- |