azelastine hydrochloride
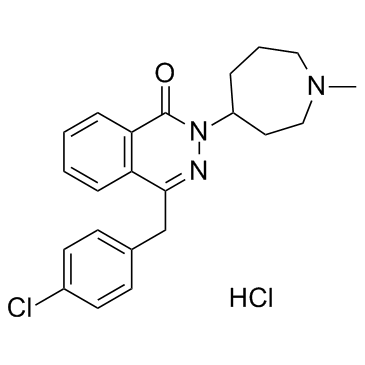
azelastine hydrochloride structure
|
Common Name | azelastine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 79307-93-0 | Molecular Weight | 418.359 | |
| Density | 1.25 g/cm3 | Boiling Point | 533.9ºC at 760 mmHg | |
| Molecular Formula | C22H25Cl2N3O | Melting Point | 225-229ºC | |
| MSDS | USA | Flash Point | 276.7ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of azelastine hydrochlorideAzelastine HCl is a potent, second-generation, selective, histamine antagonist.Target: Histamine ReceptorAzelastine is a selective H(1)-receptor antagonist that inhibits histamine release and interferes with activation of several other mediators of allergic inflammation. Azelastine can inhibit CHMCs activation and release of IL-6, tryptase, and histamine. On an equimolar basis, azelastine was a more potent inhibitor than olopatadine [1]. Topical azelastine progressively improved itching and conjunctival redness in PAC patients compared to placebo and was at least as effective as levocabastine. Rapid relief is consistent with H(1)-receptor antagonist action, while continued improvement up to 6 weeks may be consistent with mechanisms involving other mediators of allergic inflammation [2]. Azelastine nasal spray was reported to control all rhinitis symptoms, including nasal congestion, regardless of rhinitis diagnosis during the 2-week study period. Patients with seasonal allergic rhinitis and patients with seasonal allergic rhinitis plus nonallergic triggers were identified as patient types most likely to respond to azelastine nasal spray [3]. |
| Name | azelastine hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Azelastine HCl is a potent, second-generation, selective, histamine antagonist.Target: Histamine ReceptorAzelastine is a selective H(1)-receptor antagonist that inhibits histamine release and interferes with activation of several other mediators of allergic inflammation. Azelastine can inhibit CHMCs activation and release of IL-6, tryptase, and histamine. On an equimolar basis, azelastine was a more potent inhibitor than olopatadine [1]. Topical azelastine progressively improved itching and conjunctival redness in PAC patients compared to placebo and was at least as effective as levocabastine. Rapid relief is consistent with H(1)-receptor antagonist action, while continued improvement up to 6 weeks may be consistent with mechanisms involving other mediators of allergic inflammation [2]. Azelastine nasal spray was reported to control all rhinitis symptoms, including nasal congestion, regardless of rhinitis diagnosis during the 2-week study period. Patients with seasonal allergic rhinitis and patients with seasonal allergic rhinitis plus nonallergic triggers were identified as patient types most likely to respond to azelastine nasal spray [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.25 g/cm3 |
|---|---|
| Boiling Point | 533.9ºC at 760 mmHg |
| Melting Point | 225-229ºC |
| Molecular Formula | C22H25Cl2N3O |
| Molecular Weight | 418.359 |
| Flash Point | 276.7ºC |
| Exact Mass | 417.137482 |
| PSA | 38.13000 |
| LogP | 5.03740 |
| Stability | Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |
|
A qualitative method for prediction of amine oxidation in methanol and water.
J. Pharm. Sci. 104(4) , 1409-20, (2015) We have developed a predictive method, based on quantum chemical calculations, that qualitatively predicts N-oxidation by hydrogen peroxides in drug structures. The method uses linear correlations of ... |
|
|
Design and characterization of chitosan-alginate microspheres for ocular delivery of azelastine.
Pharm. Dev. Technol. 19(7) , 813-23, (2014) The use of mucoadhesive biopolymers is one of the best approaches to prolong the drug residence inside the cul-de-sac, consequently increasing the bioavailability. Thus, the focus of this work was to ... |
|
|
Adapalene inhibits the activity of cyclin-dependent kinase 2 in colorectal carcinoma.
Mol. Med. Report. 12 , 6501-8, (2015) Cyclin-dependent kinase 2 (CDK2) has been reported to be overexpressed in human colorectal cancer; it is responsible for the G1‑to‑S‑phase transition in the cell cycle and its deregulation is a hallma... |
| Azelastine hydrochloride |
| 1(2H)-Phthalazinone, 4-[(4-chlorophenyl)methyl]-2-(hexahydro-1-methyl-1H-azepin-4-yl)-, hydrochloride (1:1) |
| 4-((4-Chlorophenyl)methyl)-2-(hexahydro-1-methyl-1H-azepin-4-yl)-1(2H)-phthalazinone hydrochloride |
| 4-((4-Chlorophenyl)methyl)-2-(hexahydro-1-methyl-1H-azepin-4-yl)phthalazin-1(2H)-one hydrochloride |
| UNII:0L591QR10I |
| 1(2H)-phthalazinone, 4-[(4-chlorophenyl)methyl]-2-(hexahydro-1-methyl-1H-azepin-4-yl)-, monohydrochloride |
| 4-(4-Chlorobenzyl)-2-(1-methyl-4-azepanyl)-1(2H)-phthalazinone hydrochloride (1:1) |
| 4-(4-chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride |
| azelastinum [INN_la] |
| 4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride (1:1) |
| MFCD00242783 |
| 4-(4-Chlorbenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-onhydrochlorid |
| 4-(4-chlorobenzyl)-2-(1-méthylazépan-4-yl)phtalazin-1(2H)-one chlorhydrate |
| Azelastine (hydrochloride) |