Aluminumnitratenonahydrate
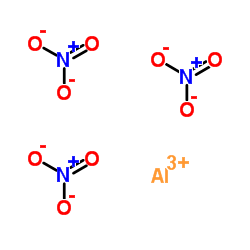
Aluminumnitratenonahydrate structure
|
Common Name | Aluminumnitratenonahydrate | ||
|---|---|---|---|---|
| CAS Number | 7784-27-2 | Molecular Weight | 212.996 | |
| Density | 1.25 | Boiling Point | 100°C/760mmHg | |
| Molecular Formula | AlN3O9 | Melting Point | 73 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS05 |
Signal Word | Danger | |
| Name | Aluminium nitrate nonahydrate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.25 |
|---|---|
| Boiling Point | 100°C/760mmHg |
| Melting Point | 73 °C(lit.) |
| Molecular Formula | AlN3O9 |
| Molecular Weight | 212.996 |
| Exact Mass | 212.944992 |
| PSA | 289.71000 |
| LogP | 0.61110 |
| Index of Refraction | 1.54 |
| InChIKey | SWCIQHXIXUMHKA-UHFFFAOYSA-N |
| SMILES | O.O.O.O.O.O.O.O.O.O=[N+]([O-])[O-].O=[N+]([O-])[O-].O=[N+]([O-])[O-].[Al+3] |
| Stability | Strong oxidizer - contact with combustible material may lead to fire. Incompatible with water, most common metals, organics. Moisture-sensitive. |
| Water Solubility | 64 g/100 mL (25 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H318 |
| Precautionary Statements | P280-P305 + P351 + P338 + P310 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36;R8 |
| Safety Phrases | S17-S26-S36 |
| RIDADR | UN 1438 5.1/PG 3 |
| WGK Germany | 1 |
| RTECS | BD1050000 |
| Packaging Group | III |
| Hazard Class | 5.1 |
| HS Code | 28342980 |
|
~% 
Aluminumnitrate... CAS#:7784-27-2 |
| Literature: Gmelin Handbook: N: MVol.4, 7.6, page 1002 - 1006 |
|
~% 
Aluminumnitrate... CAS#:7784-27-2 |
| Literature: Angewandte Chemie, , vol. 69, p. 781 - 781 Angewandte Chemie, , vol. 73, p. 388 - 393 |
| HS Code | 28342980 |
|---|
|
Multivariate optimization of process parameters in the synthesis of calcined Ca‒Al (NO3) LDH for defluoridation using 3(3) factorial, central composite and Box-Behnken design.
J. Environ. Sci. Health. A. Tox. Hazard. Subst. Environ. Eng. 51 , 86-96, (2015) Response surface methodology was applied for the first time in the optimization of the preparation of layered double hydroxide (LDH) for defluoridation. The influence of three vital process parameters... |
|
|
The metal-organic framework HKUST-1 as efficient sorbent in a vortex-assisted dispersive micro solid-phase extraction of parabens from environmental waters, cosmetic creams, and human urine.
Talanta 139 , 13-20, (2015) Three metal-organic frameworks (MOFs), specifically HKUST-1, MOF-5, and MIL-53(Al), have been synthetized, characterized, studied and compared in a vortex-assisted dispersive micro-solid-phase extract... |
|
|
Visible light functioning photocatalyst based on Al2O3 doped Mn3O4 nanomaterial for the degradation of organic toxin.
Nanoscale Res. Lett. 10 , 355, (2015) Al2O3 doped Mn3O4 nanomaterial was synthesized by low-temperature stirring method and applied as a catalyst for the degradation of organic pollutants under solar light for prospective environmental ap... |
| Aluminium trinitrate |
| Aluminum nitrate hydrate (1:3:9) |
| Nitricacid,al |
| Aluminium nitrate no |
| MFCD00149132 |
| AluminiumNitrate9H2OAcs |
| AluminiumNitrateAr |
| UNII:8MC6621V1H |
| ALUMINIUM STANDARD |
| ALUMINUM NITRATE 9H2O |
| Aluminum (III) nitrate (1:3) |
| Aluminum trinitrate |
| Aluminium nitrate hydrate (1:3:9) |
| ALUMINUM NITRATE,HYDROUS |
| ALUMINIUM NITRATE 9H2O |
| Aluminum trinitrate nonahydrate |
| EINECS 236-751-8 |
| Aluminium nitrate |
| luminum nitrate |
| Aluminumnitratenonahydrate |
 CAS#:7429-90-5
CAS#:7429-90-5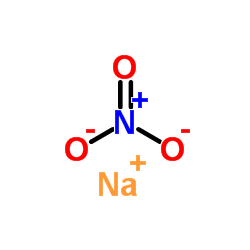 CAS#:7631-99-4
CAS#:7631-99-4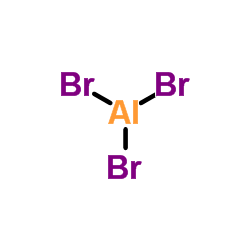 CAS#:7727-15-3
CAS#:7727-15-3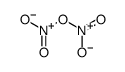 CAS#:10102-03-1
CAS#:10102-03-1
