Ebelactone A
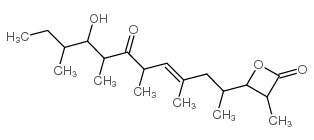
Ebelactone A structure
|
Common Name | Ebelactone A | ||
|---|---|---|---|---|
| CAS Number | 76808-16-7 | Molecular Weight | 338.48200 | |
| Density | 1.011g/cm3 | Boiling Point | 462.8ºC at 760 mmHg | |
| Molecular Formula | C20H34O4 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 152.8ºC | |
| Name | ebelactone a |
|---|---|
| Synonym | More Synonyms |
| Density | 1.011g/cm3 |
|---|---|
| Boiling Point | 462.8ºC at 760 mmHg |
| Molecular Formula | C20H34O4 |
| Molecular Weight | 338.48200 |
| Flash Point | 152.8ºC |
| Exact Mass | 338.24600 |
| PSA | 63.60000 |
| LogP | 3.76870 |
| Index of Refraction | 1.483 |
| InChIKey | WOISDAHQBUYEAF-QIQXJRRPSA-N |
| SMILES | CCC(C)C(O)C(C)C(=O)C(C)C=C(C)CC(C)C1OC(=O)C1C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | RQ7730000 |
|
Structural studies on ebelactone A and B, esterase inhibitors produced by actinomycetes.
J. Antibiot. 35 , 1495-1499, (1982)
|
|
|
Biosynthetic studies of ebelactone A and B by 13C NMR spectrometry.
J. Antibiot. 35 , 1670, (1982) Biosynthetic pathways of ebelactone A and B were studied by 13C NMR spectroscopy. By using 13C labeled compounds as precursors it was determined that ebelactone A was derived from one molecule of acet... |
|
|
Effects of ebelactone B, a lipase inhibitor, on intestinal fat absorption in the rat.
J. Enzym. Inhib. 10(1) , 57-63, (1996) Ebelactones A and B, natural products from Streptomyces aburaviensis are potent inhibitors of pancreatic lipase. Lipase is the key enzyme required for the absorption of dietary triglycerides (TG). Ebe... |
| 3,11-DIHYDROXY-2,4,6,8,10,12-HEXAMETHYL-9-OXO-6-TETRADECENOIC ACID 1,3-LACTONE |
| 3,11-DIHYDROXY-2,4,6,8,10,12-HEXAMETHYL-9-OXO-6-TETRADECANOIC-1,3-LACTONE |
| ebelactone a microbial |
| 3,11-DIHYDROXY-2,4,6,8,10,12-HEXAMETHYL-9-OXO-6-TETRADECENOIC 1,3-LACTONE |
| 3,11-DIHYDROXY-2,4,6,8,10,12-HEXAMETHYL-9-OXO-6-TETRADECEN-3-OLIDE |
| 3,11-DIHYDROXY-2,4,5,6,10,12-HEXAMETHYL-9-OXO-6-TETRADECENOIC 1,3-LACTONE |