Dimethyl methylphosphonate
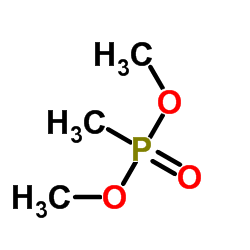
Dimethyl methylphosphonate structure
|
Common Name | Dimethyl methylphosphonate | ||
|---|---|---|---|---|
| CAS Number | 756-79-6 | Molecular Weight | 124.076 | |
| Density | 1.145 | Boiling Point | 181 ºC | |
| Molecular Formula | C3H9O3P | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 156 ºF | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
| Name | Dimethyl Methylphosphonate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.145 |
|---|---|
| Boiling Point | 181 ºC |
| Molecular Formula | C3H9O3P |
| Molecular Weight | 124.076 |
| Flash Point | 156 ºF |
| Exact Mass | 124.028931 |
| PSA | 45.34000 |
| LogP | -0.78 |
| Vapour Pressure | 1.2±0.3 mmHg at 25°C |
| Index of Refraction | 1.413 |
| Stability | Stability Combustible. Incompatible with strong oxidizing agents, strong bases. May soften some rubbers or plastics. Hydrolyzes slowly in contact with water. |
| Water Solubility | >=10 g/100 mL at 21 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H319-H340-H361f |
| Precautionary Statements | P201-P305 + P351 + P338-P308 + P313 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R36;R46 |
| Safety Phrases | S53-S26-S45 |
| RIDADR | 2810 |
| WGK Germany | 2 |
| RTECS | SZ9120000 |
|
Tandem differential mobility spectrometry with ion dissociation in air at ambient pressure and temperature.
Analyst 140(9) , 2995-3002, (2015) Proton-bound dimers were dissociated to protonated monomers in air at ambient pressure and temperature using electric fields of ultrahigh Field Asymmetric Ion Mobility Spectrometry (ultraFAIMS) with t... |
|
|
Synthetic (+)-terrein suppresses interleukin-6/soluble interleukin-6 receptor induced-secretion of vascular endothelial growth factor in human gingival fibroblasts.
Bioorg. Med. Chem. 22(19) , 5338-44, (2014) Interleukin (IL)-6 is a proinflammatory cytokine that performs a wide variety of biological functions, including important roles in the progression of chronic inflammatory diseases such as periodontal... |
|
|
Facility monitoring of chemical warfare agent simulants in air using an automated, field-deployable, miniature mass spectrometer.
Rapid Commun. Mass Spectrom. 25(10) , 1437-44, (2011) Vapors of four chemical warfare agent (CWA) stimulants, 2-chloroethyl ethyl sulfide (CEES), diethyl malonate (DEM), dimethyl methylphosphonate (DMMP), and methyl salicylate (MeS), were detected, ident... |
| MFCD00008349 |
| Dimethyl methylphosphonate |
| EINECS 212-052-3 |
| Methanephosphonic acid, dimethyl ester |
| Phosphonic acid, P-methyl-, dimethyl ester |
| [methoxy(methyl)phosphoryl]oxymethane |
| DMMP |