Aclarubicin hydrochloride
Modify Date: 2025-08-21 10:47:09
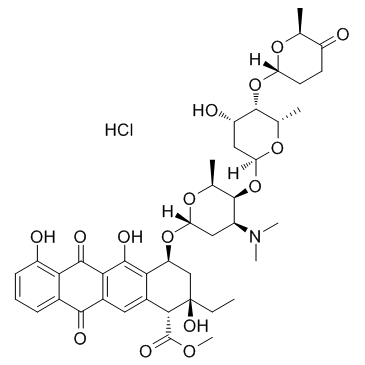
Aclarubicin hydrochloride structure
|
Common Name | Aclarubicin hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 75443-99-1 | Molecular Weight | 848.329 | |
| Density | N/A | Boiling Point | 897.7ºC at 760 mmHg | |
| Molecular Formula | C42H54ClNO15 | Melting Point | 151-153℃ | |
| MSDS | N/A | Flash Point | 496.7ºC | |
Use of Aclarubicin hydrochlorideAclacinomycin A hydrochloride (Aclarubicin hydrochloride), a fluorescent molecule, is an effective anthracycline chemotherapeutic agent for hematologic cancers and solid tumors. Accumulates efficiently in the mitochondria of living human cells and leads to mitochondrial dysfunction[1]. |
| Name | methyl (1R,2R,4S)-4-[(2R,4S,5S,6S)-4-(dimethylamino)-5-[(2S,4S,5S,6S)-4-hydroxy-6-methyl-5-[(2R,6S)-6-methyl-5-oxooxan-2-yl]oxyoxan-2-yl]oxy-6-methyloxan-2-yl]oxy-2-ethyl-2,5,7-trihydroxy-6,11-dioxo-3,4-dihydro-1H-tetracene-1-carboxylate,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Aclacinomycin A hydrochloride (Aclarubicin hydrochloride), a fluorescent molecule, is an effective anthracycline chemotherapeutic agent for hematologic cancers and solid tumors. Accumulates efficiently in the mitochondria of living human cells and leads to mitochondrial dysfunction[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Aclacinomycin A (Acla) results in an yellow solution. Aclacinomycin A could efficiently enter living cells and emit fluorescence in situ. Aclacinomycin A reveals that 10 μM Aclacinomycin A for 15 min is sufficient for clear detection of fluorescence in most of the cultured cells. Aclacinomycin A treatment (10 μM Acla for 15 min) results in approximately 20% dead (or nearly dead) cells[1]. Aclacinomycin A (Aclarubicin) effectively induces incorporation of exon 7 into SMN2 transcripts from the endogenous gene in type I SMA fibroblasts as well as into transcripts from a SMN2 minigene in the motor neuron cell line NSC34. In type I fibroblasts, treatment results in an increase in SMN protein and gems to normal levels[2]. Aclarubicin is shown to inhibit binding of topoisomerase II (Y to its DNA substrate in vitro and increases the amount of extractable topoisomerase II α[3]. |
| References |
| Boiling Point | 897.7ºC at 760 mmHg |
|---|---|
| Melting Point | 151-153℃ |
| Molecular Formula | C42H54ClNO15 |
| Molecular Weight | 848.329 |
| Flash Point | 496.7ºC |
| Exact Mass | 847.318176 |
| PSA | 217.05000 |
| LogP | 3.95970 |
| Storage condition | 2-8°C |
| Water Solubility | Soluble in DMSO or DMF at 25mg/ml |
| Hazard Codes | T: Toxic; |
|---|---|
| Risk Phrases | 23/24/25 |
| Safety Phrases | 36/37/39-45 |
| RIDADR | UN 3249 |
| RTECS | QI9283500 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| Aclarubicin HCl |
| Aclacin |
| 1-Naphthacenecarboxylic acid, 2-ethyl-1,2,3,4,6,11-hexahydro-2,5,7-trihydroxy-6,11-dioxo-4-[[2,3,6-trideoxy-4-O-[2,6-dideoxy-4-O-[(2R,6S)-tetrahydro-6-methyl-5-oxo-2H-pyran-2-yl]-α-L-lyxo-hexopyra nosyl]-3-(dimethylamino)-α-L-lyxo-hexopyranosyl]oxy]-, methyl ester, (1R,2R,4S)-, hydrochloride (1:1) |
| Aclacinomycin A hydrochloride |
| Aclarubicin hydrochloride |
| methyl (1R,2R,4S)-2-ethyl-2,5,7-trihydroxy-6,11-dioxo-4-{[2,3,6-trideoxy-4-O-{2,6-dideoxy-4-O-[(2R,6S)-6-methyl-5-oxotetrahydro-2H-pyran-2-yl]-a-L-lyxo-hexopyranosyl}-3-(dimethylamino)-a-L-lyxo-hexopyranosyl]oxy}-1,2,3,4,6,11-hexahydrotetracene-1-carboxylate hydrochloride (1:1) |
| 1-naphthacenecarboxylic acid, 2-ethyl-1,2,3,4,6,11-hexahydro-2,5,7-trihydroxy-6,11-dioxo-4-[[2,3,6-trideoxy-4-O-[2,6-dideoxy-4-O-[(2R,6S)-tetrahydro-6-methyl-5-oxo-2H-pyran-2-yl]-a-L-lyxo-hexopyranosyl]-3-(dimethylamino)-a-L-lyxo-hexopyranosyl]oxy]-, methyl ester, (1R,2R,4S)-, hydrochloride (1:1) |
| Methyl (1R,2R,4S)-2-ethyl-2,5,7-trihydroxy-6,11-dioxo-4-{[2,3,6-trideoxy-4-O-{2,6-dideoxy-4-O-[(2R,6S)-6-methyl-5-oxotetrahydro-2H-pyran-2-yl]-α-L-lyxo-hexopyranosyl}-3-(dimethylamino)-α-L-lyx o-hexopyranosyl]oxy}-1,2,3,4,6,11-hexahydro-1-tetracenecarboxylate hydrochloride (1:1) |