3-Iodobenzonitrile
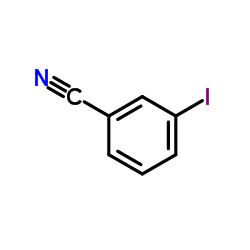
3-Iodobenzonitrile structure
|
Common Name | 3-Iodobenzonitrile | ||
|---|---|---|---|---|
| CAS Number | 69113-59-3 | Molecular Weight | 229.018 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 260.1±23.0 °C at 760 mmHg | |
| Molecular Formula | C7H4IN | Melting Point | 40-43 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 111.1±22.6 °C | |
| Name | 3-Iodobenzonitrile |
|---|---|
| Synonym | More Synonyms |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 260.1±23.0 °C at 760 mmHg |
| Melting Point | 40-43 °C(lit.) |
| Molecular Formula | C7H4IN |
| Molecular Weight | 229.018 |
| Flash Point | 111.1±22.6 °C |
| Exact Mass | 228.938828 |
| PSA | 23.79000 |
| LogP | 2.75 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.661 |
| InChIKey | BGARPMGQRREXLN-UHFFFAOYSA-N |
| SMILES | N#Cc1cccc(I)c1 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S36/37/39-S26-S37/39 |
| RIDADR | 3439 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2926909090 |
|
~89% 
3-Iodobenzonitrile CAS#:69113-59-3 |
| Literature: Kim, Jinho; Stahl, Shannon S. ACS Catalysis, 2013 , vol. 3, # 7 p. 1652 - 1656 |
|
~81% 
3-Iodobenzonitrile CAS#:69113-59-3 |
| Literature: Malet-Sanz, Laia; Madrzak, Julia; Holvey, Rhian S.; Underwood, Toby Tetrahedron Letters, 2009 , vol. 50, # 52 p. 7263 - 7267 |
|
~% 
3-Iodobenzonitrile CAS#:69113-59-3 |
| Literature: US4579848 A1, ; |
|
~% 
3-Iodobenzonitrile CAS#:69113-59-3 |
| Literature: Journal of Organic Chemistry, , vol. 55, # 11 p. 3552 - 3555 |
|
~% 
3-Iodobenzonitrile CAS#:69113-59-3 |
| Literature: Journal of the American Chemical Society, , vol. 88, # 14 p. 3318 - 3327 |
|
~%
Detail
|
| Literature: Journal of the American Chemical Society, , vol. 104, # 14 p. 3917 - 3923 |
|
~87% 
3-Iodobenzonitrile CAS#:69113-59-3 |
| Literature: Clark, James H.; Jones, Craig W.; Duke, Catherine V. A.; Miller, Jack M. Journal of Chemical Research, Miniprint, 1989 , # 8 p. 1745 - 1758 |
|
~43% 
3-Iodobenzonitrile CAS#:69113-59-3
Detail
|
| Literature: Journal of the Chemical Society, Perkin Transactions 2: Physical Organic Chemistry (1972-1999), , p. 1167 - 1174 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2926909090 |
|---|---|
| Summary | HS:2926909090 other nitrile-function compounds VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Dopamine D3 receptor antagonists: the quest for a potentially selective PET ligand. Part 3: Radiosynthesis and in vivo studies.
Bioorg. Med. Chem. Lett. 19 , 5056-5059, (2009) Compound 1 is a potent and selective antagonist of the dopamine D(3) receptor. With the aim of developing a carbon-11 labeled ligand for the dopamine D(3) receptor, 1 was selected as a potential PET p... |
|
|
Synthesis of tetrachloroisophthalo-[14C]-nitrile. Davies PE.
J. Labelled Comp. Radiopharm. 21(3) , 285-292, (1984)
|
|
|
Synthesis of Chiral Amino Acid Anilides by Ligand-Free Copper-Catalyzed Selective N-Arylation of Amino Acid Amides Dong J, et al.
Adv. Synth. Catal. 355(4) , 692-696, (2013)
|
| MFCD00079762 |
| 3-Iodobenzonitrile |
| Benzonitrile,3-iodo |
| m-iodobenzonitrile |
| 3-iodobenzenecarbonitrile |
| m-cyanophenyl iodide |
| Benzonitrile, 3-iodo- |
| 3-cyanophenyl iodide |
| 1-Cyano-3-iodobenzene |
| 3-iodo-benzonitrile |

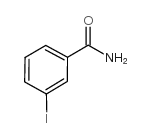
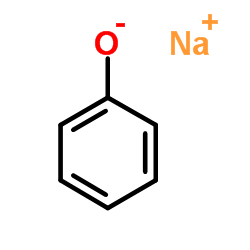
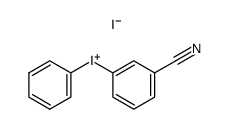
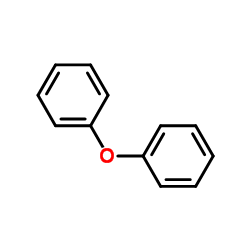

 CAS#:108697-88-7
CAS#:108697-88-7 CAS#:171290-53-2
CAS#:171290-53-2 CAS#:368-77-4
CAS#:368-77-4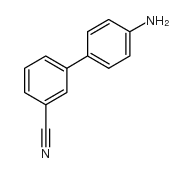 CAS#:443998-73-0
CAS#:443998-73-0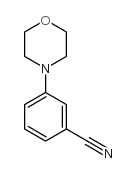 CAS#:204078-31-9
CAS#:204078-31-9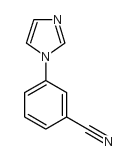 CAS#:25699-85-8
CAS#:25699-85-8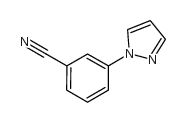 CAS#:25699-82-5
CAS#:25699-82-5 CAS#:873-62-1
CAS#:873-62-1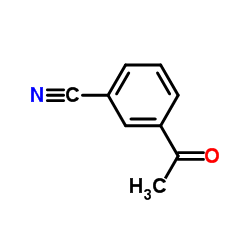 CAS#:6136-68-1
CAS#:6136-68-1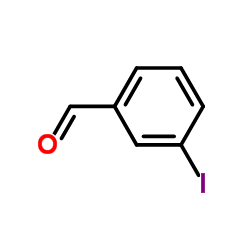 CAS#:696-41-3
CAS#:696-41-3
