Altholactone
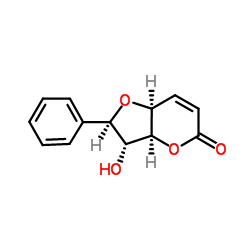
Altholactone structure
|
Common Name | Altholactone | ||
|---|---|---|---|---|
| CAS Number | 65408-91-5 | Molecular Weight | 232.23 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 490.4±45.0 °C at 760 mmHg | |
| Molecular Formula | C13H12O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 196.1±22.2 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of AltholactoneGoniothalenol is a styryl lactone that can be isolated from Goniothalamus griffithii. Goniothalenol exhibits cytatoxic activity against A2780, HCT-8, KB and MCF-7 cell lines[1]. |
| Name | (2R,3R,3aS,7aS)-3-hydroxy-2-phenyl-2,3,3a,7a-tetrahydrofuro[3,2-b]pyran-5-one |
|---|---|
| Synonym | More Synonyms |
| Description | Goniothalenol is a styryl lactone that can be isolated from Goniothalamus griffithii. Goniothalenol exhibits cytatoxic activity against A2780, HCT-8, KB and MCF-7 cell lines[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 490.4±45.0 °C at 760 mmHg |
| Molecular Formula | C13H12O4 |
| Molecular Weight | 232.23 |
| Flash Point | 196.1±22.2 °C |
| Exact Mass | 232.073563 |
| PSA | 55.76000 |
| LogP | 1.00 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.598 |
| Storage condition | ?20°C |
|
Stereoselective total synthesis of bioactive styryllactones (+)-goniofufurone, (+)7-epi-goniofufurone, (+)-goniopypyrone, (+)-goniotriol, (+)-altholactone, and (-)-etharvensin.
J. Org. Chem. 73(1) , 2-11, (2008) Stereoselective total synthesis of biologically active styryllactones 7-epi-goniofufurone, goniofufurone, goniopypyrone, goniotriol, altholactone, and etharvensin was achieved in high overall yields f... |
|
|
The cytotoxicity of naturally occurring styryl lactones.
Phytomedicine 13(3) , 181-6, (2006) We extracted and isolated three natural styryl lactones from Goniothalamus griffithii Hook f. Thoms and investigated their cytotoxicity on a panel of three hepatocyte cell lines, HepG2, drug resistant... |
|
|
Asymmetric synthesis of (+)-altholactone: a styryllactone isolated from various Goniothalamus species.
Chemistry 14(9) , 2842-9, (2008) The asymmetric total synthesis of (+)-altholactone (1), a member of the styryllactone family of natural products displaying cytotoxic and antitumor activities, is described. Key steps include a RAMP-h... |
| 2-phenyl-3-hydroxy-6,7-dihydro-furano-pyrone |
| (2R,3R,3aS,7aS)-3-Hydroxy-2-phenyl-2,3,3a,7a-tetrahydro-5H-furo[3,2-b]pyran-5-one |
| goniothalenol |