Pomaglumetad Methionil
Modify Date: 2025-08-25 22:49:17
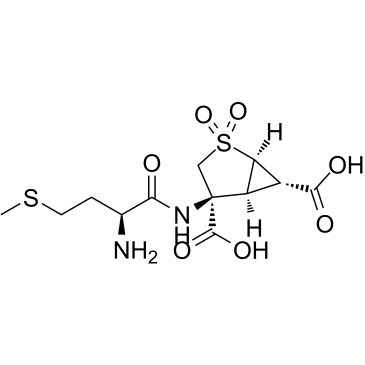
Pomaglumetad Methionil structure
|
Common Name | Pomaglumetad Methionil | ||
|---|---|---|---|---|
| CAS Number | 635318-55-7 | Molecular Weight | 235.214 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 600.3±55.0 °C at 760 mmHg | |
| Molecular Formula | C12H18N2O7S2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 316.8±31.5 °C | |
Use of Pomaglumetad MethionilLY2140023 is an orally active prodrug of LY404039. LY404039 is a selective metabotropic glutamate 2/3 receptor agonist. LY2140023 is currently for the treatment of schizophrenia[1]. |
| Name | (1R,4S,5S,6S)-4-[[(2S)-2-amino-4-methylsulfanylbutanoyl]amino]-2,2-dioxo-2λ6-thiabicyclo[3.1.0]hexane-4,6-dicarboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | LY2140023 is an orally active prodrug of LY404039. LY404039 is a selective metabotropic glutamate 2/3 receptor agonist. LY2140023 is currently for the treatment of schizophrenia[1]. |
|---|---|
| Related Catalog | |
| Target |
mGluR2 mGluR3 |
| In Vivo | LY2140023 (orally; 3, 10, and 300 mg/kg; once daily for 7 days) dose-dependent increases the levels of the dopamine metabolites dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA)[1]. Animal Model: Male Fischer rats (approximately 250 g)[1] Dosage: 3, 10, and 300 mg/kg Administration: Orally; once daily for 7 days Result: Dose-dependent increased the levels of the dopamine metabolites DOPAC and HVA. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 600.3±55.0 °C at 760 mmHg |
| Molecular Formula | C12H18N2O7S2 |
| Molecular Weight | 235.214 |
| Flash Point | 316.8±31.5 °C |
| Exact Mass | 235.015060 |
| PSA | 143.14000 |
| LogP | -2.02 |
| Vapour Pressure | 0.0±3.7 mmHg at 25°C |
| Index of Refraction | 1.661 |
| InChIKey | VOYCNOJFAJAILW-LWDQJBINSA-N |
| SMILES | CSCCC(N)C(=O)NC1(C(=O)O)CS(=O)(=O)C2C(C(=O)O)C21 |
| UNII-3V85EZ3KFQ |
| 2-Thiabicyclo[3.1.0]hexane-4,6-dicarboxylic acid, 4-amino-, 2,2-dioxide, (1R,4S,5S,6S)- |
| LY-404,039 |
| Pomaglumetad methionil |
| (1R,4S,5S,6S)-4-Amino-2-thiabicyclo[3.1.0]hexane-4,6-dicarboxylic acid 2,2-dioxide |