Miltefosine
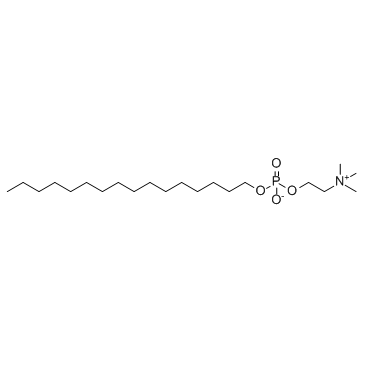
Miltefosine structure
|
Common Name | Miltefosine | ||
|---|---|---|---|---|
| CAS Number | 58066-85-6 | Molecular Weight | 407.568 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C21H46NO4P | Melting Point | 232-234ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
Use of MiltefosineMiltefosine is a broad spectrum antimicrobial, anti-leishmanial, phospholipid agent acting by inhibiting the PI3K/Akt activity. |
| Name | miltefosine |
|---|---|
| Synonym | More Synonyms |
| Description | Miltefosine is a broad spectrum antimicrobial, anti-leishmanial, phospholipid agent acting by inhibiting the PI3K/Akt activity. |
|---|---|
| Related Catalog | |
| Target |
Akt HIV-1 |
| In Vitro | Treatment of HIV-1 infected macrophages with Miltefosine inhibits the recruitment of PH-AktGFP to the plasma membrane. Since Miltefosine inhibits Akt through mimicry of the PH domain, it is likely that Miltefosine binds to PIP3, blocking the recruitment of PH-Akt to the membrane[1]. Miltefosine (HePC) inhibits protein kinase C (PKC) from NIH3T3 cells in cell-free extracts with a IC50 of about 7 µM. Inhibition is competitive with regard to phosphatidylserine with a Ki of 0.59 µM[2]. Miltefosine is an alkylphospholipid that inhibit activation of Akt. Miltefosine is a direct inhibitor of Akt, and induces dose-dependent inhibition of primary effusion lymphoma (PEL) in culture and also inhibits the downstream targets of Akt, such as mTOR, leading to reduced phosphorylation and activation of S6K and S6. Importantly, Miltefosine also inhibits Akt targets that are not part of the mTOR pathway, eg, FOXO1, and are therefore expected to have a greater therapeutic impact than mTORC1 inhibitors alone[3]. |
| In Vivo | Mice are randomized into groups of 5 and injected intraperitoneally 5 days a week with 50 mg/kg of either Miltefosine or Perifosine dissolved in PBS, or equivalent volume of vehicle (PBS). Both Miltefosine and Perifosine inhibit the growth rate of tumors compared with vehicle-treated mice. By day 14 after treatment, there is an approximately 50% decrease in average tumor volume in Perifosine- and Miltefosine-treated mice, compared with vehicle-treated mice (P<0.04). Tumor growth is also significantly retarded (P<0.04 for Perifosine and P≤0.055 for Miltefosine by linear mixed-effects model analysis). Immunohistochemical analyses display an overall reduction in staining for phosphorylated ribosomal S6 protein in tumor sections from Miltefosine- and Perifosine-treated mice compared with the PBS-treated mice. This reduced phosphorylation correlated with the delay in tumor progression in drug-treated animals[3]. |
| Kinase Assay | Levels of enzymatically active caspase-3 are quantified using the ApoAlert Caspase Fluorescent assay kit. Briefly, 1×106 BC-1 PEL cells are treated with 50 μM Miltefosine, 50 μM Perifosine, or 20 nM NVP-BEZ235, as well as the respective vehicle controls. Cells are harvested and lysed 12 hours later. Equivalent micrograms of cell lysate for all samples are incubated with a fluorogenic caspase-3 substrate (DEVD-AFC). Cleavage of DEVD by caspase-3 releases AFC, the fluorescence of which is measured using a FLUOstar OPTIMA fluorometer, with excitation and emission filter wavelengths set to 400 and 505 nm, respectively[3]. |
| Cell Assay | NIH3T3 cells are grown in DMEM supplemented with 10% FCS in a humidified atmosphere of 95% air with 5% CO2. Cells are plated on 35-mm culture dishes (6-well plates) at 0.5-0.8×105 cells/well. Growth is established for 18-24 h and the cell number of representative wells is determined (time 0). The experiments are started by addition of fresh prepared solution of Miltefosine at given concentrations to the cells or equal volumes of Tris-HCI to control cells. After incubation for 60 h, cells are counted with an electronic counter. Cellular multiplication is calculated[2]. |
| Animal Admin | Mice[3] PEL cells are washed in ice-cold phosphate buffered saline, counted, and diluted in 100 μL of PBS mixed with 100 μL of growth factor-depleted Matrigel. A total of 1×105 to 7.5×105 BC-1 cells are injected subcutaneously into the right flank of NOD.CB17-Prkdcscid/J or CB17-Prkdcscid/J mice. The mice are monitored on alternate days for development of palpable tumors (2 mm3), at which point drug or vehicle treatments are initiated, and are administered either intraperitoneally (Perifosine) or by oral gavage (Rosiglitazone, NVP-BEZ235) 5 days a week. Groups of 5 to 7 mice are used to generate PEL tumors and treated with either vehicle or drug cocktail. Each biologic experiment is repeated multiple times. For Rosiglitazone, 0.25% methylcellulose is used as vehicle, and 30 mg/kg or 60 mg/kg Rosiglitazone is suspended in methylcellulose. For Perifosine and Miltefosine, PBS is used as a vehicle and 50 mg/kg Perifosine or Miltefosine is dissolved in PBS. For NVP-BEZ235, the compound is dissolved in a 1:9 vol/vol mixture of 1-methyl-2-pyrrolidone and polyethylene glycol 300. A dose of 40 mg/kg NVP-BEZ235 or equal volume of the vehicle is administered. Tumor diameters are measured using digital calipers, and tumor volume is calculated. The tumors are excised and fixed in formalin. Statistical analyses are performed using linear model fit by maximum likelihood with individual animals treated as random effect. Rats[4] Male Sprague-Dawley rats (weight 270-290 g) are divided into five groups (n=5). Rats in the treatment groups are administered a single 10 mg/kg oral dose of Miltefosine (MFS) either as an aqueous solution or MFS-LNCs dispersion by gastric gavage. This dose is equivalent to the 20 mg/kg Miltefosine dose administered to mice in the preclinical study after correction for rats. Following administration, blood samples are collected via the orbital plexus under anesthesia at time intervals of 0.5, 1, 2, 4, 7, 10, 24, 48, 72 and 216 h in Eppendorf tubes containing EDTA. Blood samples are then centrifuged immediately at 4000 rpm for 10 min. Plasma samples are frozen and maintained at -80°C pending analysis. |
| References |
| Melting Point | 232-234ºC |
|---|---|
| Molecular Formula | C21H46NO4P |
| Molecular Weight | 407.568 |
| Exact Mass | 407.316437 |
| PSA | 68.40000 |
| LogP | 3.58 |
| Storage condition | room temp |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H334 |
| Precautionary Statements | P261-P301 + P310-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | 22-43 |
| Safety Phrases | 36/37 |
| RIDADR | UN 2811 6.1 / PGIII |
| RTECS | KH2890000 |
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Alkylphosphocholines and curcumin induce programmed cell death in cutaneous T-cell lymphoma cell lines.
Leuk. Res. 38(1) , 49-56, (2014) While most patients with early-stage cutaneous T-cell lymphomas (CTCL) have a very good prognosis, the survival of patients with extensive tumour stage and visceral involvement remains extremely poor ... |
|
|
In vitro drug susceptibility of Leishmania infantum isolated from humans and dogs.
Exp. Parasitol. 135(1) , 36-41, (2013) Visceral leishmaniasis (VL) caused by parasites of Leishmania donovani complex is a severe human disease which often leads to death if left untreated. Domestic dogs are the main reservoir hosts for zo... |
| Miltex |
| Hexadecyl 2-(trimethylammonio)ethyl phosphate |
| 2-[[(Hexadecyloxy)hydroxyphosphinyl]oxy]-N,N,N-trimethylethanaminium Inner Salt |
| Ethanaminium, 2-[[(hexadecyloxy)hydroxyphosphinyl]oxy]-N,N,N-trimethyl-, inner salt |
| MIL |
| Miltefosine |
| hexadecyl 2-(trimethylazaniumyl)ethyl phosphate |
| HPC [VAN] |
| Miltefosine [BAN:INN] |
| hexadecylphosphocholine |
| Impavido |
| MFCD00133396 |
| Hexadecyl 2-(N,N,N-trimethylamino)ethyl phosphate |